This site uses cookies. By continuing to browse the site you are agreeing to our use of cookies.
All
Agriculture and Farming
Agriculture and Food News -- ScienceDaily
CropLife
Farming Today
Modern Farmer
National Sustainable Agriculture Coalition
We Found a Work Around to Trump Defunding Sci...
Apr 22, 2025 0
In Rural England, Farming Equipment Has Becom...
Apr 22, 2025 0
How Maryland Hit Its 30x30 Goal
Apr 22, 2025 0
We Found a Work Around to Trump Defunding Sci...
Apr 22, 2025 0
In Rural England, Farming Equipment Has Becom...
Apr 22, 2025 0
How Maryland Hit Its 30x30 Goal
Apr 22, 2025 0
South Carolina Says PFAS-Contaminated Farmlan...
Apr 21, 2025 0
The Ultimate Guide to Guest Post: Boost Your ...
Mar 8, 2025 0
All
All Stories
All Stories
BioPharma Dive - Latest News
Breaking World Pharma News
Drugs.com - Clinical Trials
Drugs.com - FDA MedWatch Alerts
Drugs.com - New Drug Approvals
Drugs.com - Pharma Industry News
FDA Press Releases RSS Feed
Federal Register: Food and Drug Administration
News and press releases
Pharmaceuticals news FT.com
PharmaTimes World News
Stat
What's new
Scientists achieve record-breaking growth in ...
Apr 20, 2025 0
Popular diabetes medications, including GLP-1...
Apr 20, 2025 0
Potential treatment for Parkinson's usin...
Apr 20, 2025 0
Researchers develop an LSD analogue with pote...
Apr 20, 2025 0
All
Breaking DefenseFull RSS Feed – Breaking Defense
DefenceTalk
Defense One - All Content
Military Space News
NATO Latest News
The Aviationist
War is Boring
War on the Rocks
Informal meeting of NATO Ministers of Foreign...
Apr 17, 2025 0
Ceremony to mark the 70th anniversary of Germ...
Apr 17, 2025 0
Military Committee in Chiefs of Defence Session
Apr 16, 2025 0
Joint press statement
Apr 16, 2025 0
All
Advanced Energy Materials
CleanTechnica
Energy | FT
Energy | The Guardian
EnergyTrend
Nature Energy
NYT > Energy & Environment
PV-Tech
RSC - Energy Environ. Sci. latest articles
Utility Dive - Latest News
Lessons learned from Los Angeles’s just energ...
Apr 22, 2025 0
Ultrafast Na+ Diffusion Enabled by Defective ...
Apr 22, 2025 0
Flux Upcycling of Degraded Layered Cathodes t...
Apr 22, 2025 0
Ambipolar Interfacial Molecule for Enhancing ...
Apr 22, 2025 0
In Situ Diffraction and Ex Situ Transmission ...
Apr 22, 2025 0
Lessons learned from Los Angeles’s just energ...
Apr 22, 2025 0
Green Solutions to Fight Louisiana Flooding
Apr 22, 2025 0
A Funeral Director Brought Wind Power to Rock...
Apr 22, 2025 0
- Contact
- Agriculture
- Automotive
- Beauty
-
Biopharma
- All
- All Stories
- All Stories
- BioPharma Dive - Latest News
- Breaking World Pharma News
- Drugs.com - Clinical Trials
- Drugs.com - FDA MedWatch Alerts
- Drugs.com - New Drug Approvals
- Drugs.com - Pharma Industry News
- FDA Press Releases RSS Feed
- Federal Register: Food and Drug Administration
- News and press releases
- Pharmaceuticals news FT.com
- PharmaTimes World News
- Stat
- What's new
- Defense
- Energy & Water
- Fashion
- Food & Beverage
- Healthcare
- Legal
- Manufacturing
- Luxury
- Medical Devices
- Mining
- Real Estate
- Retail
- Science Journals
- Transport & Logistics
- Travel & Hospitality









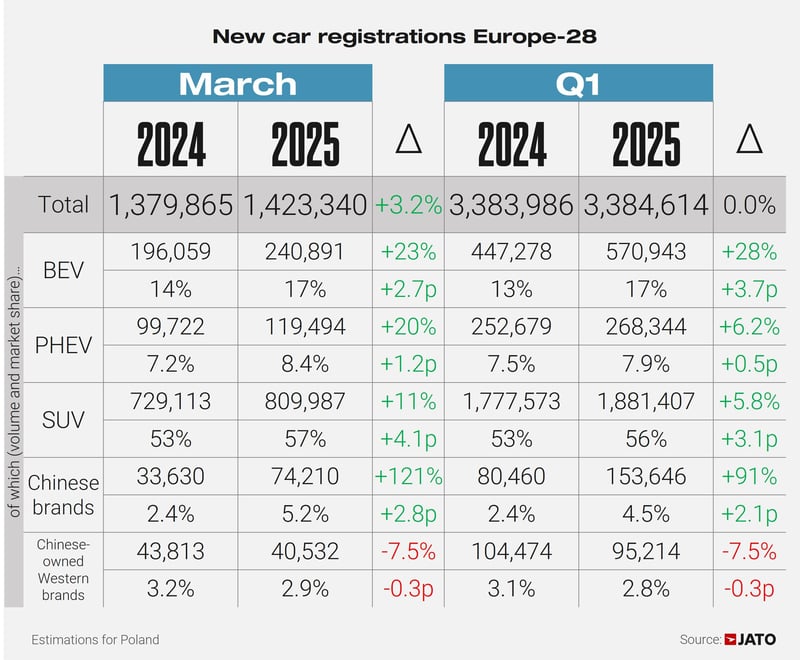


























































































































































.jpg)
.jpg)
.jpg)
















