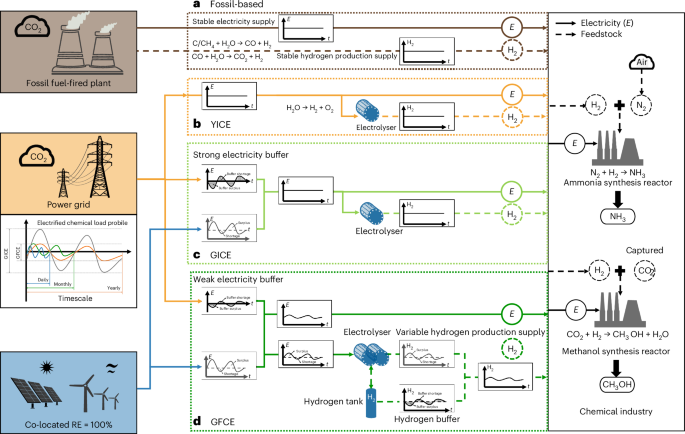
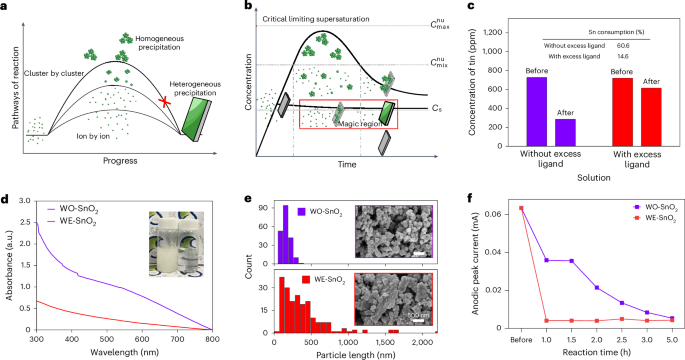
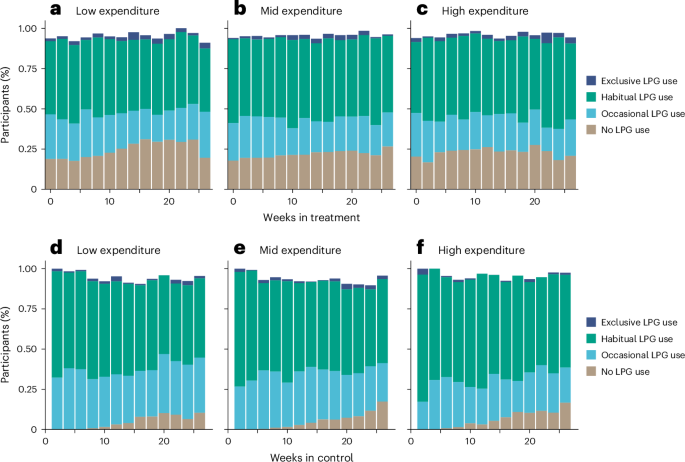
U.S. Food and Drug Administration Updates Camzyos (mavacamten) Label to Reduce Echocardiography Monitoring Requirements and Contraindications
PRINCETON, N.J.-- April 17, 2025 -- (BUSINESS WIRE)-- Bristol Myers Squibb (NYSE: BMY) today announced that the U.S. Food and Drug Administration (FDA) has updated the U.S. Prescribing Information for Camzyos (mavacamten, 2.5 mg, 5 mg, 10 mg, 15 mg...