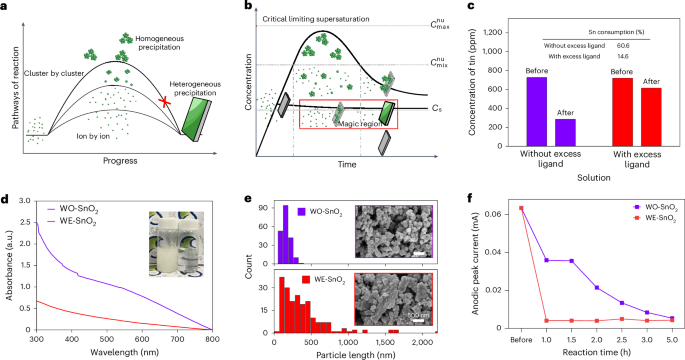
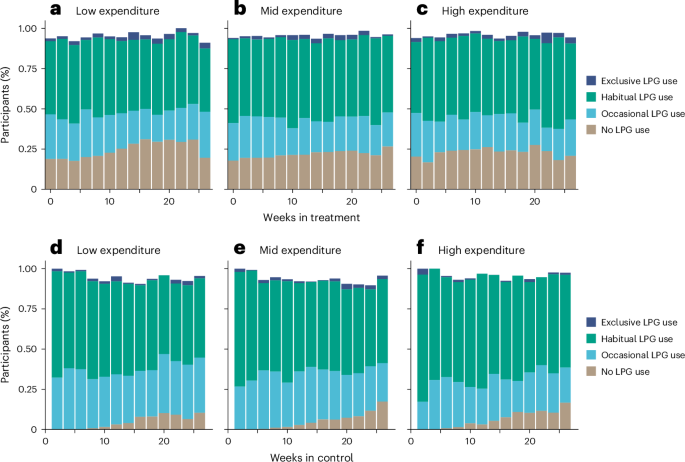
U.S. FDA Approves Third Indication of Nubeqa (darolutamide) for Patients with Advanced Prostate cancer
Berlin, June 3, 2025 – Bayer announced today that the U.S. Food and Drug Administration (FDA) has approved its oral androgen receptor inhibitor (ARi) Nubeqa (darolutamide) in combination with androgen deprivation therapy (ADT) for use in...