In Situ Diffraction and Ex Situ Transmission X‐Ray Microscopy Studies of Solid‐State Upcycling for NMC Cathodes
Advanced Energy Materials, EarlyView.

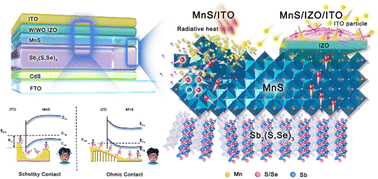
Description of attached TOC figure: Phase evolution observed from in situ X-ray diffraction experiments during upcycling of NMC622 is shown in a contour plot in the center. 3D nickel XANES maps generated using variable energy transmission X-ray microscopy are presented on the left and right to capture the change in the distribution of nickel during upcycling when using an sulfate and acetate precursor, respectively.
Abstract
Upcycling of recycled LiNi0.6Mn0.2Co0.2O2 (NMC622) cathodes offers an economical route to produce cathode materials with increased energy density (i.e., LiNi0.8Mn0.1Co0.1O2, NMC811) that meet the performance needs of present-day electric vehicles. In this work, solid-state upcycling of NMC622 via calcination with Ni(OH)2 and LiOH was monitored using in situ synchrotron powder X-ray diffraction measurements. Sequential Rietveld refinements indicate that the calcination proceeds by initially converting Ni(OH)2 to a rocksalt NiO phase followed by lithiation of NiO to form LiNiO2 (LNO), with both NMC and LNO phases present in nearly equal proportions at the calcination endpoint. Variable-energy transmission X-ray microscopy tomograms of upcycled samples reveal that the NMC and LNO domains are intermixed at sub-micron length scales. Depth-dependent analysis of multi-elemental fitting maps matches the expected NMC811 composition at the secondary particle level and indicates that transition metal diffusion is not limited by the secondary particle size.




































































































































































.jpg)
.jpg)
.jpg)






