Microneedle Array‐Based Dermal Interstitial Fluid Biopsy for Cancer Diagnosis: Advances and Challenges
Advanced Healthcare Materials, EarlyView.

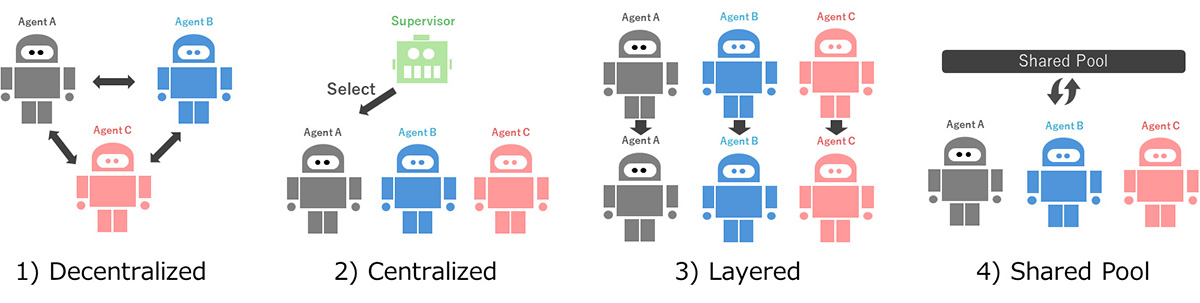
Microneedle-based Dermal ISF liquid biopsy offers a practical cancer diagnostic option for LMICs. New microneedle designs and integrated sensor technologies showed potential for early detection; biosensors may enhance accuracy and enable real-time applications. MN-ISF-based diagnosis requires continuous innovation and validation to overcome technical limitations and achieve wider acceptance for standardized clinical use.
Abstract
Current early cancer diagnostic technologies, such as imaging, molecular tests, endoscopic techniques, and biopsies, face considerable challenges in low-and middle-income countries (LMICs) due to high costs, procedural complexity, and limited resource access. Microneedle-based liquid biopsy for skin interstitial fluid (ISF) offers a practical and minimally invasive alternative for cancer diagnosis in these settings. This review systematically examines ISF liquid biopsy methods for their effectiveness in capturing cancer biomarkers directly from the skin and assesses their potential to address diagnostic needs in low-resource environments. Recent innovations in microneedle design and ISF underscore their potential in enabling early, accessible cancer detection tailored to LMICs' needs. Additionally, integrating artificial intelligence (AI) for data interpretation is proposed as a way to enhance diagnostic accuracy and enable real-time point-of-care (POC) applications. Collectively, these advances illustrate a flexible, scalable model for accessible cancer diagnostics, with significant implications for improving early detection and healthcare quality in resource-limited environments.