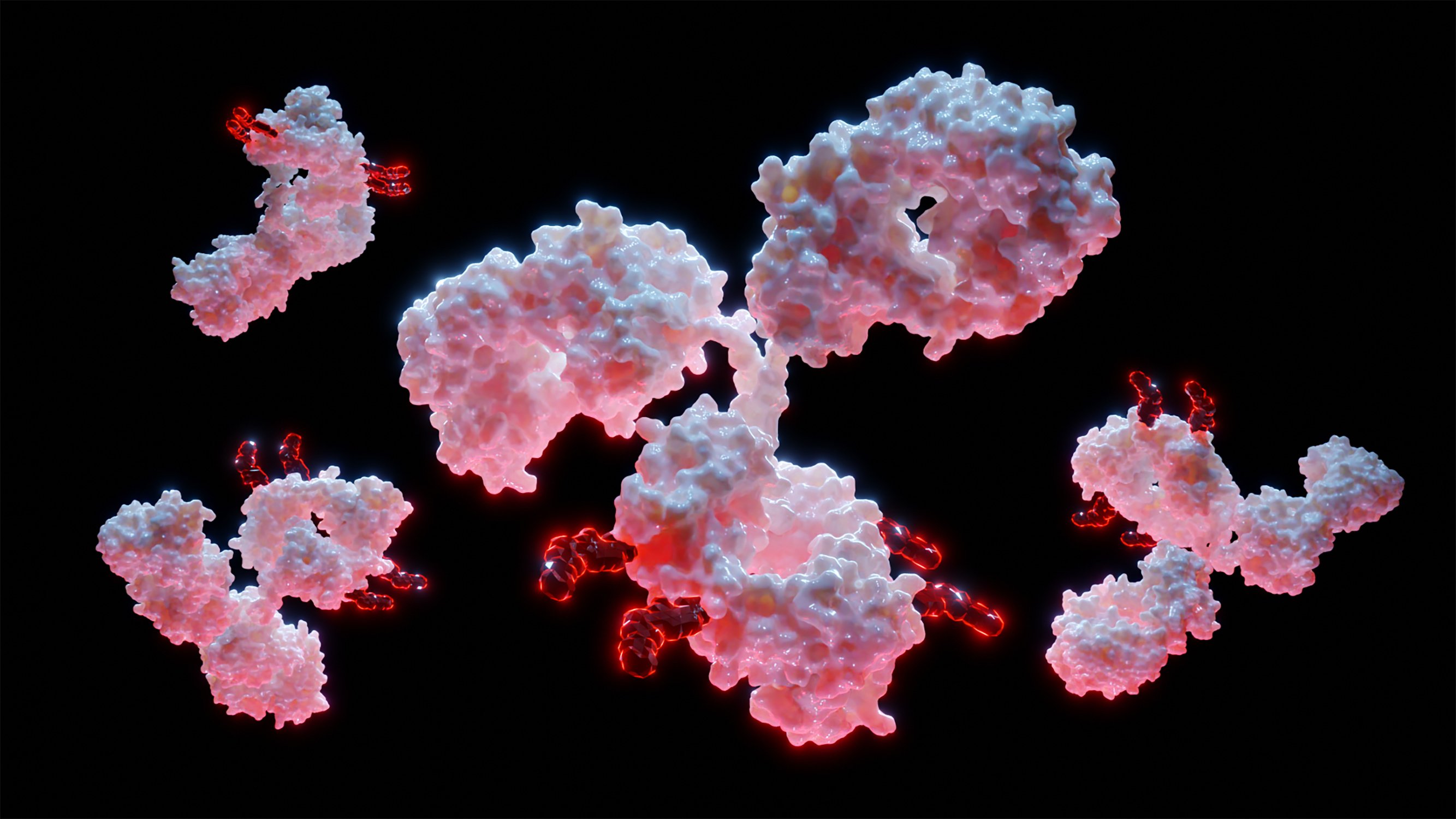
Intrinsically Pro‐Apoptotic Gold Nanoclusters for Optical Tracing and Inhibition of Solid Tumors
Advanced Healthcare Materials, Volume 14, Issue 15, 10 June, 2025.

A novel class of fluorescent quasi-molecular gold nanoclusters stabilized with a cross-linked and fluorinated glycopolymer is presented. The nanocluster's near infrared emission facilitated imaging of its tumor uptake and enabled tracking of rapid renal clearance. They also caused mitochondrial membrane depolarization toward oxidative stress mediated apoptotic cell death with significant tumor inhibition in preclinical mouse model.
Abstract
Intrinsically theranostic metal nanoclusters are rare unless the stabilizing ligands exhibit therapeutic properties. A promising class of quasi-molecular, near-infrared (NIR) emitting, cytotoxic gold nanoclusters, coined as AXE (Au eXcitable and Eliminable) stabilized through terminal thioester groups on fluorinated, and crosslinked polymers, is presented for simultaneous bioimaging & therapy. Nano Variable Temperature-Electrospray ionization mass spectrometry analysis of these aqueous stable nanoclusters revealed 5 to 7 core gold atoms, with SAXS measurement confirming average size to be under 1 nm, consistent with the theoretical maximum for few atom planar gold clusters. Despite its small size, AXE exhibits a remarkable Stoke shift of ≈470 nm and emission range spanning 700 to 1100 nm. Fluorination notably enhanced the quantum yield by up to twofold, attributed to charge transfer from the fluorinated monomer to the gold core, as indicated by Löwdin charge distribution analysis. The AXE nanocluster demonstrated dose-dependent pro-apoptotic effects on cancer cells while sparing normal cells at lower concentrations. Preclinical evaluation in a breast tumor model confirmed its anticancer efficacy, with intravenous and intraperitoneal administrations significantly inhibiting tumor growth and controlling lung metastasis, surpassing the clinical standard, doxorubicin.



















































































![Israel drama and good news for the F-35: Paris Air Show Day 1 [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/IMG_1805-scaled-e1750092662883.jpg?#)














![[Updated] Sudden Deployment of Dozens of U.S. Air Force Tankers Raises Questions](https://theaviationist.com/wp-content/uploads/2025/03/Stratotanker100Years_2-e1750080240327.jpg)