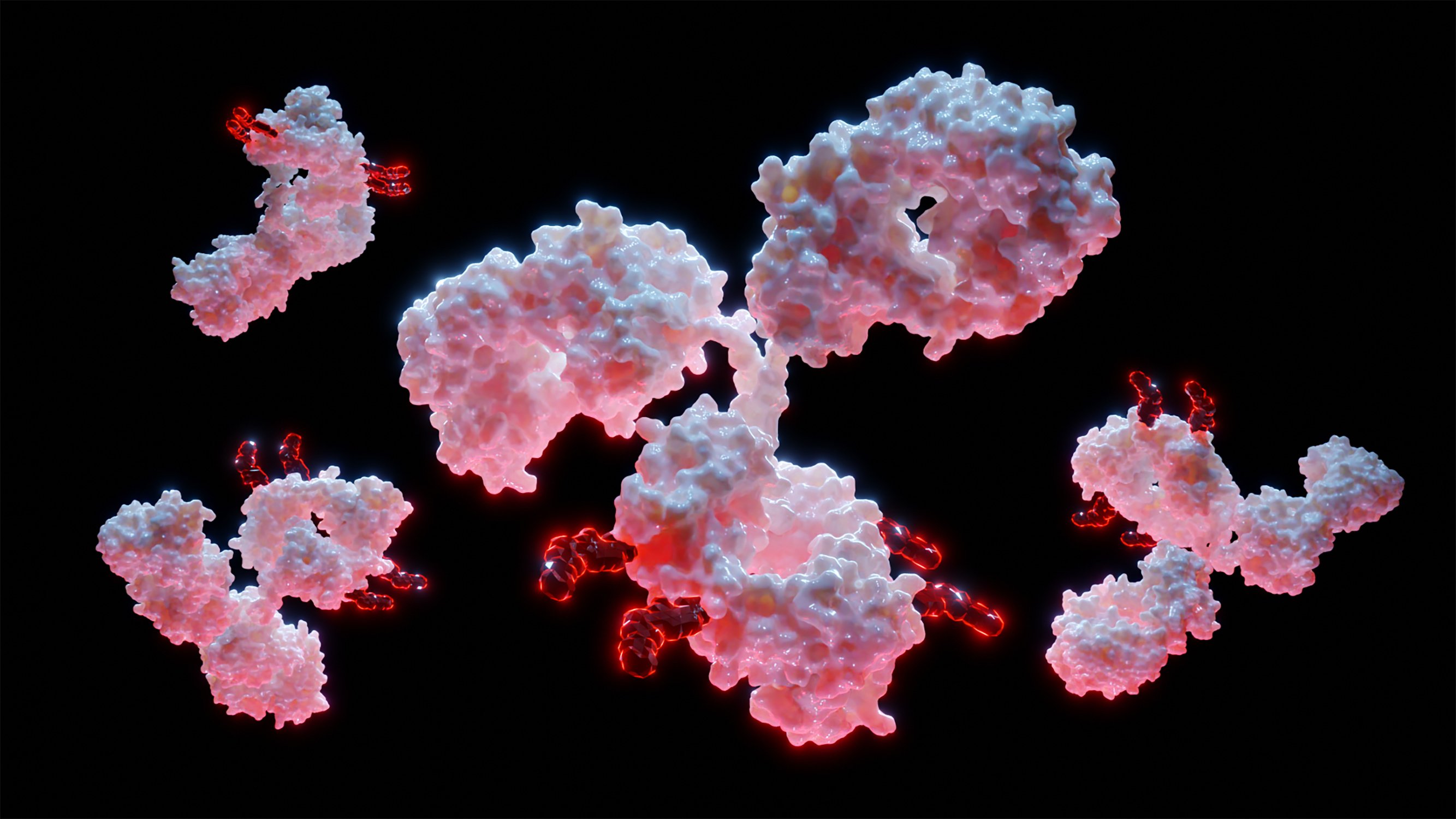
FDA Approves Andembry (garadacimab-gxii) for Prophylaxis to Prevent Attacks of Hereditary Angioedema
KING OF PRUSSIA, Pa., June 16, 2025 /PRNewswire/ -- Global biotechnology leader CSL today announced the U.S. Food and Drug Administration (FDA) approved Andembry (garadacimab-gxii), the only treatment targeting factor XIIa for prophylactic use to...


















































































![[Updated] U.S. Air Force Mobilizes F-22s and F-35s as Situation in Middle East Escalates](https://theaviationist.com/wp-content/uploads/2025/06/F-22_F-35_CENTCOM-top.jpg)




![Israel drama and good news for the F-35: Paris Air Show Day 1 [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/IMG_1805-scaled-e1750092662883.jpg?#)












![[Updated] Sudden Deployment of Dozens of U.S. Air Force Tankers Raises Questions](https://theaviationist.com/wp-content/uploads/2025/03/Stratotanker100Years_2-e1750080240327.jpg)