Fluoroalkane Engineered Magnetic Vectors Unlock the Potential of Gasdermin in Vivo Delivery for Pyroptosis Induced Cancer Therapy
Advanced Healthcare Materials, Volume 14, Issue 15, 10 June, 2025.

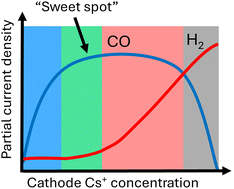
Fluoroalkane-modified iron oxide nanoparticles (IONPs) enable proteins with different molecular weights and isoelectric points to escape endosomes and lysosomes and improve intracellular delivery efficiency, particularly in magnetic fields. Applying this nanoplatform to active GSDMA3 eliminates the need for additional chemical conjugation or activation steps to trigger pyroptosis, facilitating significant tumor suppression and robust immune activation in both in vitro and in vivo models.
Abstract
Pyroptosis, a programmed necrotic cell death mediated by gasdermin, can activate strong immune responses and serve as a potential target for cancer therapy. Nevertheless, the relatively large molecular size and negative surface charge of gasdermin impede them from effectively intracellular delivery and directly inducing pyroptosis. Here, a cytosolic protein delivery system, fluorinated iron oxide nanoparticles (FIONPs) is reported, which can self-assemble with active gasdermin A3 protein (GSDMA3) via noncovalent interactions and effectively trigger pyroptosis in 4T1 cells. It is proved that the delivery system is versatile for various cargo proteins (ribonuclease A, saporin, β-galactosidase, and bovine serum albumin) with different isoelectric points and molecular weights, without compromising their biological activity in vitro. What's more, under magnetic drive, FIONPs facilitate active transport of GSDMA3 in vivo, further augmenting tumor suppression and immune response. Overall, magnetic-driven FIONPs provide an effective delivery system for intracellular protein transductions, and the application of the delivery system reveals that direct delivery of GSDMA3 significantly elicits robust antitumor immunity via the induction of pyroptosis.


















































































![Israel drama and good news for the F-35: Paris Air Show Day 1 [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/IMG_1805-scaled-e1750092662883.jpg?#)
![[Updated] Sudden Deployment of Dozens of U.S. Air Force Tankers Raises Questions](https://theaviationist.com/wp-content/uploads/2025/03/Stratotanker100Years_2-e1750080240327.jpg)