Enhanced Zinc Deposition and Dendrite Suppression in Aqueous Zinc‐Ion Batteries Via Citric Acid‐Aspartame Electrolyte Additives
Advanced Energy Materials, EarlyView.

The illustration features two gate god standing on either side of a gate, symbolizing the synergistic effect of aspartame and citric acid. Outside the gate, there are weeds and small stone piles, signifying the potential occurrence of hydrogen evolution reaction, the generation of by-products, and the growth of zinc dendrites without the presence of these two additives. Inside the gate, the pathway is clear and smooth, indicating that the additives provide a strong protective effect on the zinc anode, preventing these issues.
Abstract
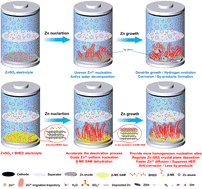
Despite the advantages of low cost, safety, and environmental friendliness, aqueous zinc-ion batteries (AZIBs) encounter challenges such as zinc dendrite formation, severe side reactions, and electrolyte instability. Many effective additives exhibit limited solubility in water, thus reducing their practical application potential. In this study, a dissolution-promoting strategy is proposed by introducing citric acid (CA) to enhance the dissolution of aspartame (APM), resulting in a zinc sulfate electrolyte. Simulations and experiments indicate that CA regulates both the solvation structure of Zn2+ and the pH of the electrolyte, while APM preferentially integrates into the electric double layer to form a solid electrolyte interphase with CA, thereby suppressing hydrogen evolution and side reactions. Consequently, the zinc-zinc symmetric cell exhibits an extended lifespan of over 4,500 h at 1.0 mA cm−2/1.0 mAh cm−2. As a result, the AZIBs with this electrolyte and commercial zinc foil and MnO2 exhibit enhanced rate capability and improved capacity retention (75.6%) after 2,000 cycles. This study presents a novel strategy for stabilizing zinc anodes and offers a comprehensive framework for addressing fundamental challenges in AZIBs, advancing their practical application in next-generation energy storage systems.