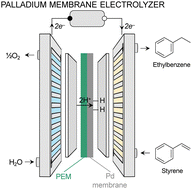
Enhanced Redox Kinetics of Aqueous I−/I2/I+ Conversion Chemistry in Hydrated Eutectic Electrolyte Over a Wide Temperature Range
Advanced Energy Materials, EarlyView.

The practical application of four-electron zinc-iodine aqueous batteries (4eZIBs) is seriously restricted by their sluggish iodine conversion kinetics and unstable zinc plating/stripping. Herein, a cost-effective hydrated eutectic electrolyte rich in organic cations is designed to enhance the reversibility and kinetics of Zn deposition and four-electron iodine conversion over a wide temperature range from −25 to 40 °C.
Abstract
Due to the continuous I−/I2/I+ redox couples, four-electron zinc-iodine aqueous batteries (4eZIBs) offer a high theoretical capacity of 422 mAh g−1. However, sluggish iodine conversion kinetics and unstable zinc plating/stripping significantly limit their widespread adoption. Here, a cost-effective hydrated eutectic electrolyte enriched with organic cations is developed to enhance the reversibility and kinetics of Zn deposition and four-electron iodine conversion across a wide temperature range. Specifically, the iodophilic choline cation (Ch+), in synergy with glycerol, significantly stabilizes I+ and enhances the redox kinetics of iodine species. Concurrently, the adsorption of Ch+ on the anode surface promotes the uniform deposition of Zn2+. Furthermore, the interaction between eutectic components and water disrupts the hydrogen bond network of free water molecules, thereby enhancing the freeze resistance of the electrolyte. Consequently, the 4eZIBs with optimized hydrated eutectic electrolyte not only demonstrate remarkable cyclability with a low-capacity decay of 0.0016% per cycle over 15 000 cycles but also exhibit excellent temperature adaptability in a wide temperature range from −25 to 40 °C. This work provides new insights into the rational design of high-performance 4eZIBs through the organic cation chemistry and optimized electrolyte structures.





































































































![[Updated] B-2 Spirit Bombers Struck Iranian Nuclear Sites](https://theaviationist.com/wp-content/uploads/2025/06/B2sGuam_2.jpg)