Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 16-19 June 2025
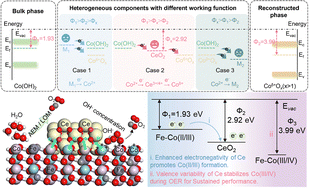
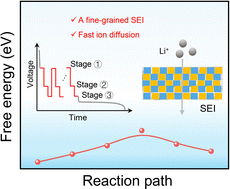
13 new medicines recommended for approvalEMA’s human medicines committee (CHMP) recommended 13 medicines for approval at its June 2025 meeting.The committee recommended granting a…, CHMP statistics Key figures from the June 2025 CHMP meeting are represented in the graphic below., CHMP statistics: Text versionJune 2025 statistics - monthly and cumulative figures for CHMP opinions and withdrawn applications:13 positive opinions on new medicines: 3 new non…, Positive recommendations on new medicines , Austedo International non-proprietary name (INN) deutetrabenazine Marketing-…, Imreplys INN sargramostim Marketing-authorisation applicant…, Ogsiveo INN nirogacestat Marketing-authorisation applicant…, Rezdiffra INN resmetirom Marketing-authorisation applicant…, Zemcelpro INN Dorocubicel / Allogeneic umbilical cord-derived CD34- cells, non-expanded…, Positive recommendations on new biosimilar medicines , Afiveg INN aflibercept Marketing-authorisation applicant…, Eiyzey INN aflibercept Marketing-authorisation applicant…, Mynzepli INN aflibercept Marketing-authorisation applicant…, Usymro INN ustekinumab Marketing-authorisation applicant…, Vgenfli INN aflibercept Marketing-authorisation applicant…, Vivlipeg INN pegfilgrastim Marketing-authorisation applicant…, Positive recommendations on new generic medicines , Emtricitabine/Rilpivirine/Tenofovir Alafenamide Viatris INN emtricitabine / rilpivirine / tenofovir alafenamide…, Nintedanib Viatris INN nintedanib Marketing-authorisation applicant…, Positive recommendations on extensions of therapeutic indications , Benlysta INN belimumab Marketing-authorisation holder…, Cabometyx INN cabozantinib Marketing-authorisation holder…, Darzalex INN daratumumab Marketing-authorisation holder…, Imbruvica INN ibrutinib Marketing-authorisation holder…, Nubeqa INN darolutamide Marketing-authorisation holder…, Sarclisa INN isatuximab Marketing-authorisation holder…, Start of re-examination of initial applications , Atropine sulfate FGK INN atropine Marketing authorisation applicant…, Kisunla INN donanemab Marketing authorisation applicant…, Start of referral , Sodium oxybate syrup and oral solution used in alcohol dependence Marketing authorisation applicant Various –…, Recommendations on extensions of therapeutic indication for one medicine intended for use outside the EU , Dapivirine Vaginal Ring 25 mg INN dapivirine Marketing authorisation applicant…
13 new medicines recommended for approvalEMA’s human medicines committee (CHMP) recommended 13 medicines for approval at its June 2025 meeting.The committee recommended granting a…, CHMP statistics Key figures from the June 2025 CHMP meeting are represented in the graphic below., CHMP statistics: Text versionJune 2025 statistics - monthly and cumulative figures for CHMP opinions and withdrawn applications:13 positive opinions on new medicines: 3 new non…, Positive recommendations on new medicines , Austedo International non-proprietary name (INN) deutetrabenazine Marketing-…, Imreplys INN sargramostim Marketing-authorisation applicant…, Ogsiveo INN nirogacestat Marketing-authorisation applicant…, Rezdiffra INN resmetirom Marketing-authorisation applicant…, Zemcelpro INN Dorocubicel / Allogeneic umbilical cord-derived CD34- cells, non-expanded…, Positive recommendations on new biosimilar medicines , Afiveg INN aflibercept Marketing-authorisation applicant…, Eiyzey INN aflibercept Marketing-authorisation applicant…, Mynzepli INN aflibercept Marketing-authorisation applicant…, Usymro INN ustekinumab Marketing-authorisation applicant…, Vgenfli INN aflibercept Marketing-authorisation applicant…, Vivlipeg INN pegfilgrastim Marketing-authorisation applicant…, Positive recommendations on new generic medicines , Emtricitabine/Rilpivirine/Tenofovir Alafenamide Viatris INN emtricitabine / rilpivirine / tenofovir alafenamide…, Nintedanib Viatris INN nintedanib Marketing-authorisation applicant…, Positive recommendations on extensions of therapeutic indications , Benlysta INN belimumab Marketing-authorisation holder…, Cabometyx INN cabozantinib Marketing-authorisation holder…, Darzalex INN daratumumab Marketing-authorisation holder…, Imbruvica INN ibrutinib Marketing-authorisation holder…, Nubeqa INN darolutamide Marketing-authorisation holder…, Sarclisa INN isatuximab Marketing-authorisation holder…, Start of re-examination of initial applications , Atropine sulfate FGK INN atropine Marketing authorisation applicant…, Kisunla INN donanemab Marketing authorisation applicant…, Start of referral , Sodium oxybate syrup and oral solution used in alcohol dependence Marketing authorisation applicant Various –…, Recommendations on extensions of therapeutic indication for one medicine intended for use outside the EU , Dapivirine Vaginal Ring 25 mg INN dapivirine Marketing authorisation applicant…





















































































![Four big takeaways to wrap up the Paris Air Show [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2023/06/FCAS-scaled-e1685718848105.jpg?#)
![The sights of Paris Air Show, one last time: Day 4 [Photos]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/20250617-helenedelacoste-Paris-Air-Show-037-scaled-e1750357690820.jpg?#)