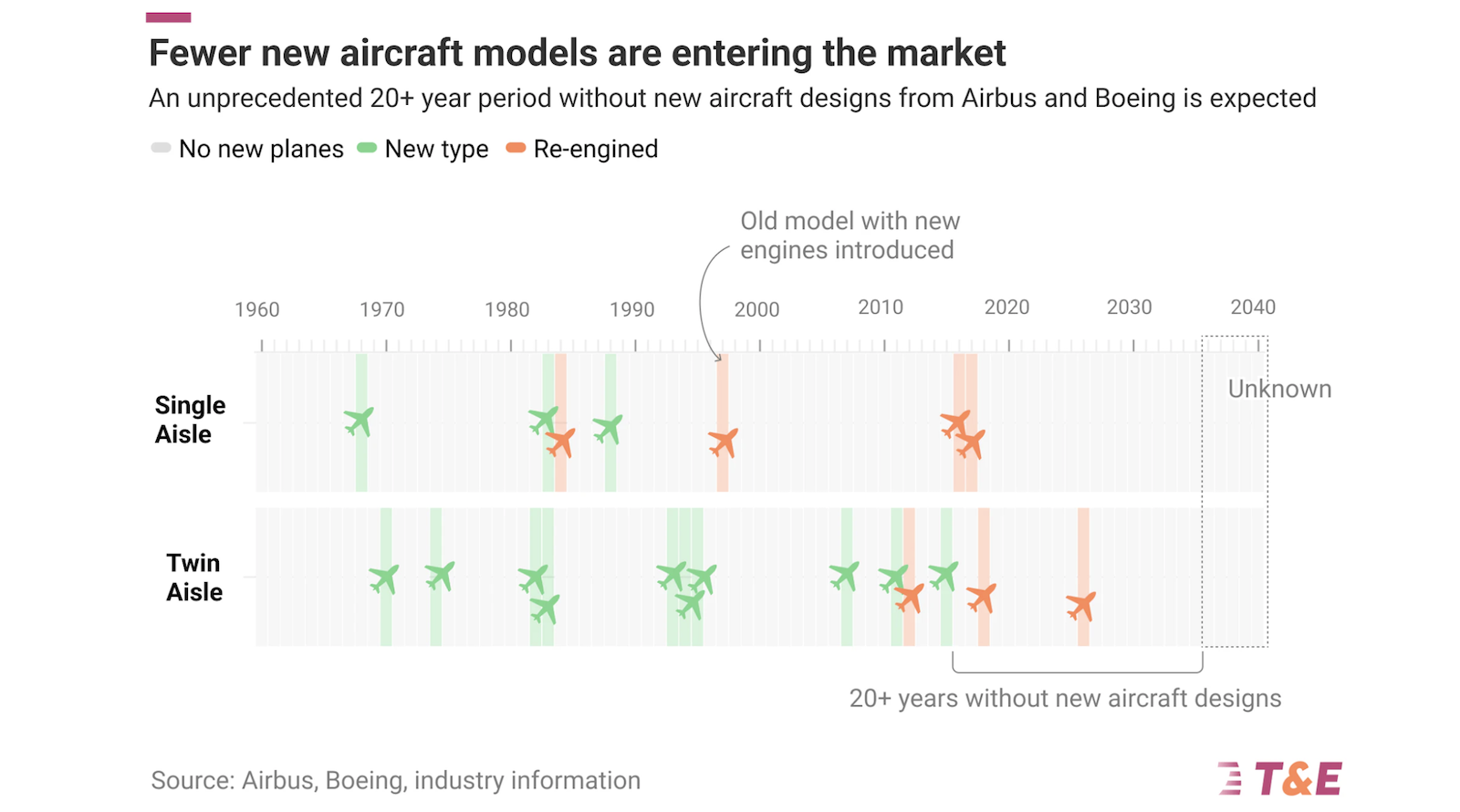
Adjustable Selectivity for CO2 Electroreduction to Ethylene or Ethanol by Regulating Interphases Between Copper and Tin Oxides
Advanced Energy Materials, Volume 15, Issue 23, June 17, 2025.

By regulating the interphases in Cu-SnO2 and Cu2O-SnO2 catalysts, it is found that Cu-SnO2 interphase exhibits outstanding advantages of CO* and COH* generation for the asymmetric coupling of CO*-COH*, leading to efficient ethanol production. At the Cu2O-SnO2 interphase, the oxygen vacancies at both sites enrich *CO intermediates for symmetrical C–C coupling to produce ethylene.
Abstract
Enhancing the selectivity of C2 products and revealing the reaction mechanisms in CO2 electroreduction reaction (CO2RR) remain challenging. Regulating the interphases in catalysts is one of the most promising pathways. Herein, the interphases between copper (Cu) and tin (Sn) oxides are regulated by controlling the degree of reduction during the self-assembly process, which exhibits obvious different selectivity to ethylene and ethanol, respectively. The interphase in Cu-SnO2 exhibits selectivity to ethanol as high as 74.6%, while the interphase in Cu2O-SnO2 shows selectivity to ethylene as high as 71.4% at –0.6 V versus RHE. In situ Fourier-transform infrared spectroscopy measurements and density functional theory calculations demonstrate that the interphase in Cu-SnO2 shows strong electron interaction, preferentially forming the key *COH intermediates for asymmetrical C─C coupling to produce ethanol. In contrast, Cu2O-SnO2 possesses oxygen vacancies at both sites, thus enriching *CO intermediates for symmetrical C─C coupling to produce ethylene at the interphase. The findings in this work offer an advanced strategy by regulating the interphases to adjust C2 selectivity in CO2RR.


























































































![The American contingent and Turkey’s autonomy goals: Paris Air Show Day 3 [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/Wednesday-Wrap.00_00_32_21.Still001.png?#)
![A look at the jets flying high above the Paris Air Show [PHOTOS]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/Rafale_02-scaled-e1750268097167.jpg?#)