STAT+: Gilead’s CEO on the approval of a powerful new drug to prevent HIV
Gilead's CEO says he's committed to making sure people around the world can get his firm's new HIV prevention shot Yeztugo.

On Wednesday, the Food and Drug Administration approved a new medicine, lenacapavir (brand name: Yeztugo) that could be the closest thing so far to an HIV vaccine: a long-acting antiviral that, given twice a year, can prevent people from contracting the virus. Researchers agree that it could be a game-changer. It is made by Gilead Sciences, whose work in HIV has made it one of the largest biotech companies.
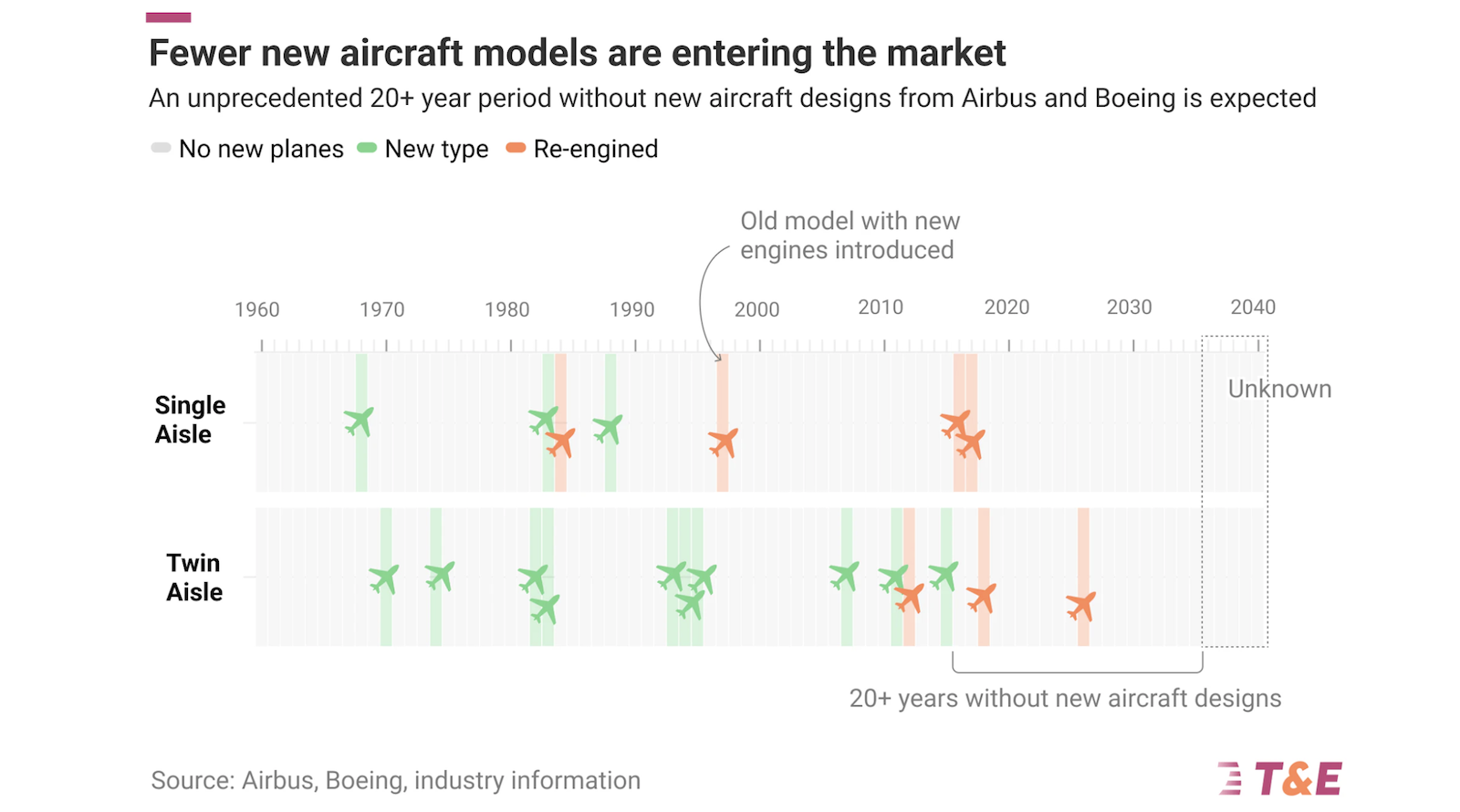
This is undoubtedly one of the most important drug approvals this year. Yet the excitement over this breakthrough has dimmed somewhat because of the climate around HIV prevention. The Trump administration has gutted federal support for HIV treatment and prevention both in the U.S. and overseas. Some domestic cuts have been rolled back, but the moves raise doubts about how Yeztugo will reach patients who need it.
Shortly before the approval, STAT caught up with Daniel O’Day, Gilead’s CEO, about how he intends to get this expensive medicine (it’s expected to cost thousands of dollars per shot) to people around the globe. A transcript of the discussion, edited for length and clarity, follows.























































































![The American contingent and Turkey’s autonomy goals: Paris Air Show Day 3 [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/Wednesday-Wrap.00_00_32_21.Still001.png?#)
![A look at the jets flying high above the Paris Air Show [PHOTOS]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/Rafale_02-scaled-e1750268097167.jpg?#)