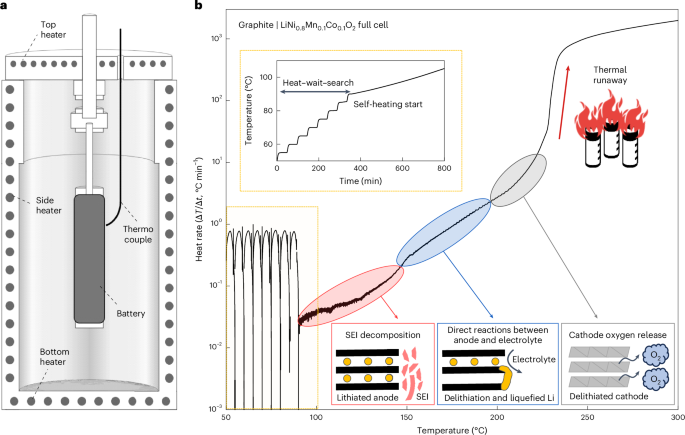
Dexcom’s FDA warning letter reveals unauthorized changes to sensors
Dexcom made a significant design change to a component used in its sensors and did not adequately validate the change, according to the warning letter.
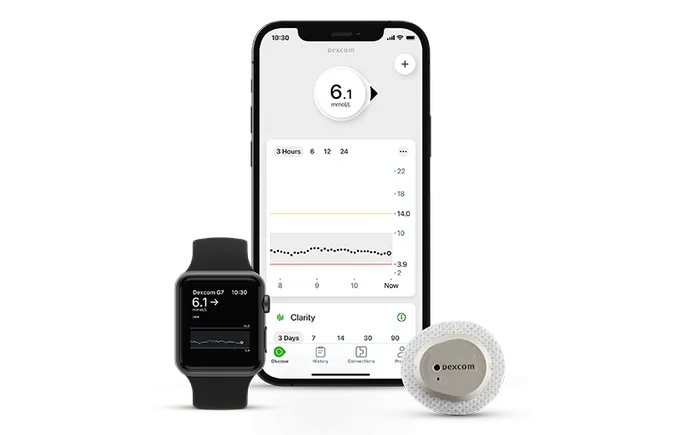
Dexcom made a significant design change to a component used in its sensors and did not adequately validate the change, according to the warning letter.