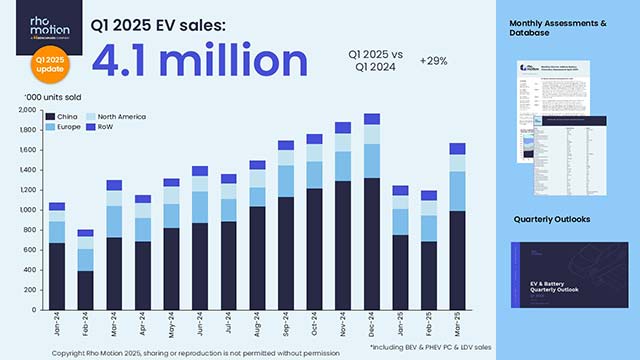
STAT+: Pharmalittle: We’re reading about RFK Jr. targeting CDC and FDA advisers, a Pfizer gene therapy program, and more
HHS Secretary Robert F. Kennedy Jr. is preparing to remove committee members who advise the government on vaccine approvals

And so, another working week will soon draw to a close. Not a moment too soon, yes? This is, you may recall, our treasured signal to daydream about weekend plans. Our agenda is, so far at least, rather modest. We have a great deal of reading to catch up on and plan to take care of sundry errands. This may leave us more time for another listening party, where the rotation may include this, this, this, this and this. And what about you? This may be an opportunity to try a new restaurant, take a long drive somewhere to enjoy the sights and unwind, or perhaps make time to reach out to someone special. Well, whatever you do, we hope you have a grand time. But be safe. Enjoy, and see you soon. …
U.S. Health and Human Services Secretary Robert F. Kennedy Jr. is preparing to remove members of the outside committees that advise the federal government on vaccine approvals and other key public health decisions, according to Politico. Kennedy plans to replace members who he perceives to have conflicts of interest, as part of a widespread effort to minimize what he has criticized as undue industry influence over the nation’s health agencies. Kennedy has long argued that drugmakers have too much sway over the approval of their products. The effort is likely to target the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, which plays a key role in setting vaccine policy. Kennedy and his top aides are also scrutinizing a host of other outside panels, including those that advise the U.S. Food and Drug Administration.
Bluebird Bio announced it would sell itself and its portfolio of gene therapies to the investment firms Carlyle and SK Capital for less than $30 million, in a deal that lets the beleaguered biotech avoid bankruptcy, STAT says. The sale to private equity marks a quiet end for a biotech that, over the course of a dizzying decade, showcased both the power of modern genetic medicine to deliver cures for devastating rare diseases and the profound challenges any company would face in turning that power into a sustainable business. For more than a decade, its technology for inserting genes into blood cells could produce jaw-dropping results, seemingly curing patients of sickle cell and beta thalassemia, both blood disorders, as well as children born with a fatal ultra-rare neurological disease called CALD. Turning those early results into treatments, though, proved a minefield. They were difficult to manufacture and it took years for Bluebird to actually show it could produce them to regulatory standards. It was also unclear if the company would be able to get the kind of reimbursement it had been seeking from governments and insurers.