Understanding the Metal‐Center Mediated Adsorption and Redox Mechanisms in a FeMn(NbTa)2O6 Columbite Material for Anion Exchange Membrane Water Electrolyzers
Advanced Energy Materials, EarlyView.

FeMn(NbTa)2O6-columbite is a sustainable and high-performance bifunctional electrocatalyst for anion exchange membrane water electrolyzers, comparable to benchmark catalysts. In situ XAS and DFT analysis show Fe/Mn sites exhibit higher OER activity due to reduced overpotentials and favorable O→OOH energetics. The Nb/Ta sites facilitate charge transfer, lower activation barriers, and increase stability. FeMn(NbTa)2O6-columbite electrocatalyst exhibits remarkable durability and efficiency, matching Pt/C-RuO₂ catalysts, offering a promising alternative for sustainable hydrogen production.
Abstract
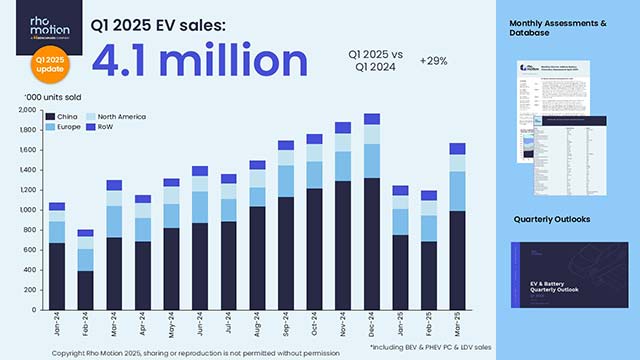
The rising demand for sustainable green hydrogen production necessitates efficient and cost-effective water-splitting electrocatalysts. Inspired by the catalytic activities of columbite-tantalite, this study combines a scalable cutting-edge synthesis approach with atomic-level structures and metal-center-mediated mechanisms to unravel its operational performance and stability. Using ad in situ X-ray absorption fine structure combined with Density Functional Theory (DFT), the results reveal distinctive valence band peaks and moderate charge transfer from Mn and Fe sites, enabling stable adsorption and reduced activation barriers. In contrast, the high-valence Nb and Ta centers at the B-sites promote favorable d-band alignment, enhancing orbital overlap with oxygen p-orbitals. This facilites electronic delocalization, lowers charge accumulation, and reduces activation barriers of intermediates species. Fe and Mn at the A-sites exhibit strong redox reactivity and optimal adsorption for OH* and O*, supporting efficient electron fransfers. Solvation effects modeled via VASPsol further stabilize key intermediates, especially O*, reducing the energy barrier for water dissociation. Notably, FeMn(NbTa)2O6-columbite catalysts stand out with a cell voltage of 1.81 V at a current density of 700 mA cm−2, compared to 40% Pt/C-RuO₂ (1.75 V) at the same current density in the anion exchange membrane water electrolyzer (AEMWE). Also, the FeMn(NbTa)2O6-columbite exhibits long-term stability at 800 mA cm−2, surpassing the benchmark 40% Pt Vulcan-RuO2 after 200 h in AEMWE. This work significantly advances current research and establishes a design rule for selecting metal compositions in the development of advanced electrocatalysts in alkaline water electrolyzers.