Role of Polymer‐Iodine Complexes on Solid‐Liquid Polysulfide Phase Transitions and Rate Capability of Lithium Sulfur Batteries
Advanced Energy Materials, Volume 15, Issue 11, March 18, 2025.

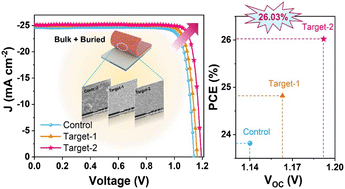
A catalytic binder system is designed by immobilizing iodine centers within a polysulfide-loving polymer, addressing the inherent sulfur loading and C-rate trade-off. In pouch cells, it achieves energy densities of 215 at 0.1C and 156 Wh kg−1 at 0.3C and 262 Wh kg−1 at Ah-level, demonstrating one of the highest-rate Li-S pouches reported in lithium-sulfur literature.
Abstract
Lithium–sulfur (Li–S) batteries are considered as a viable technology offering energy-dense electrochemical energy storage systems. However, the inherently slow reaction kinetics manifested in the slow charge and discharge characteristics constrain their real-world applications. Here, it is reported that polyiodide species held within a complex polar network of polyvinylpyrrolidone (PVP) accelerate the rate-limiting solid-liquid phase transitions both in the reduction and oxidation steps during battery cycling. Density functional theory calculations support a mechanism in which a combination of enhanced binding of polysulfides and additional energy states in the PVP-iodine-polysulfide complexes accelerates the reaction pathways mediated by inter-valance polyiodide reactions within the working voltage of Li–S batteries. These studies show that PVP-iodine (PVP-I) complexes enhance the rate capability of cells with practical sulfur loadings delivering a high areal capacity of ≈7 mAh cm−2 at the practical 0.5C rate. This advantage is demonstrated in one of the highest-rate pouches reported in Li–S literature, attaining energy densities of 215 and 156 Wh kg at 0.1C and 0.3C, respectively. The results demonstrate a subtle but powerful shift in the design of molecular binder systems, which have functional roles above and beyond the role of simply holding the active materials together.


















































































































































![Restoration of Li+ pathways in the [010] direction during direct regeneration for spent LiFePO4](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=D5EE00641D)


























.jpg)







