Human medicines European public assessment report (EPAR): Efavirenz/Emtricitabine/Tenofovir disoproxil Zentiva, efavirenz,emtricitabine,tenofovir disoproxil, Date of authorisation: 17/07/2017, Revision: 11, Status: Authorised
Human medicines European public assessment report (EPAR): Efavirenz/Emtricitabine/Tenofovir disoproxil Zentiva, efavirenz,emtricitabine,tenofovir disoproxil, Date of authorisation: 17/07/2017, Revision: 11, Status: Authorised


















































































































































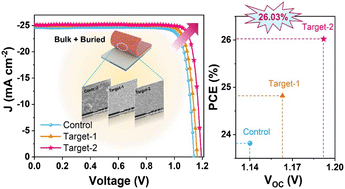
![Restoration of Li+ pathways in the [010] direction during direct regeneration for spent LiFePO4](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=D5EE00641D)


























.jpg)







