Moderna's next-gen COVID shot gets FDA backing with some limitations
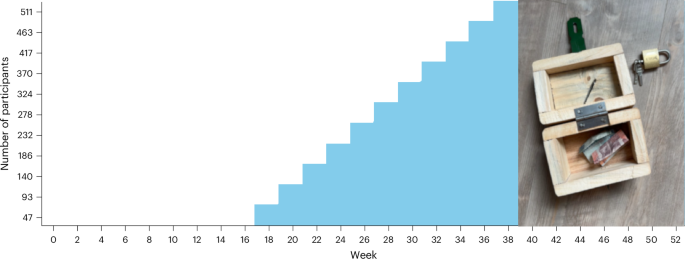
Moderna has picked up its third FDA approval with the agency's endorsement of mNEXSPIKE. The approval covers the vaccine's use in people 65 and older and those ages 12 to 64 with risk factors for severe COVID.




















































































![[Video] The Weekly Break Out Ep. 20: Pacific policy in Singapore and the UK’s new defense plan](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/Break-Out-ep-20-thumb-Play-Button.jpg?#)