Managing bicarbonate salt formation in CO<sub>2</sub> reduction electrolysers for stable operation
Nature Energy, Published online: 30 January 2025; doi:10.1038/s41560-025-01707-xObservations of salt formation in CO2 reduction electrolysers were used to propose a mechanism for salt precipitation linked to the drying of liquid droplets carrying cations and (bi)carbonate ions. A hydrophobic surface coating was used to remove droplets from the flow channels before they can dry, increasing the operational stability of the electrolyser.
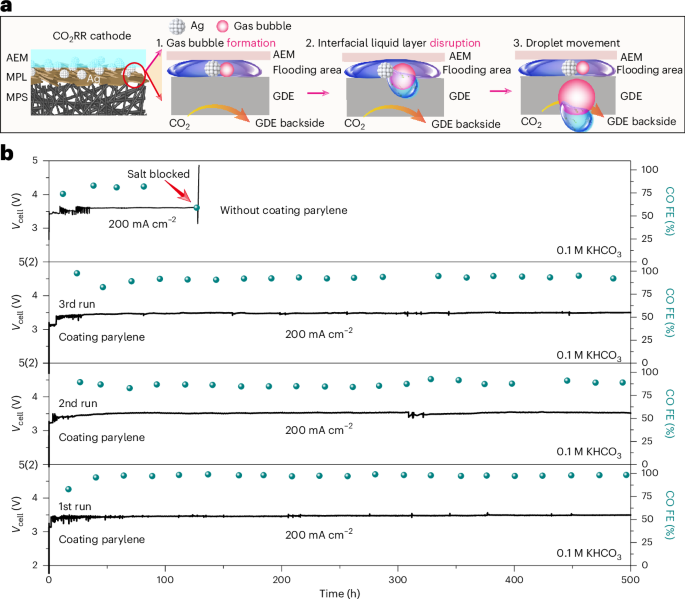
Nature Energy, Published online: 30 January 2025; doi:10.1038/s41560-025-01707-xObservations of salt formation in CO2 reduction electrolysers were used to propose a mechanism for salt precipitation linked to the drying of liquid droplets carrying cations and (bi)carbonate ions. A hydrophobic surface coating was used to remove droplets from the flow channels before they can dry, increasing the operational stability of the electrolyser.