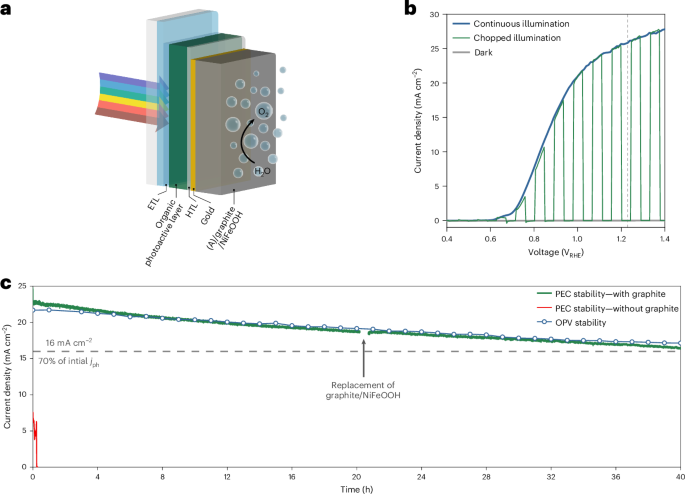
Human medicines European public assessment report (EPAR): Cosentyx, secukinumab, Date of authorisation: 14/01/2015, Revision: 40, Status: Authorised
Human medicines European public assessment report (EPAR): Cosentyx, secukinumab, Date of authorisation: 14/01/2015, Revision: 40, Status: Authorised


































































































![The sights of Avalon Air Show 2025: Day Three [PHOTOS]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/03/f-35-avalon-final-day-scaled-e1743079275404.jpg?#)