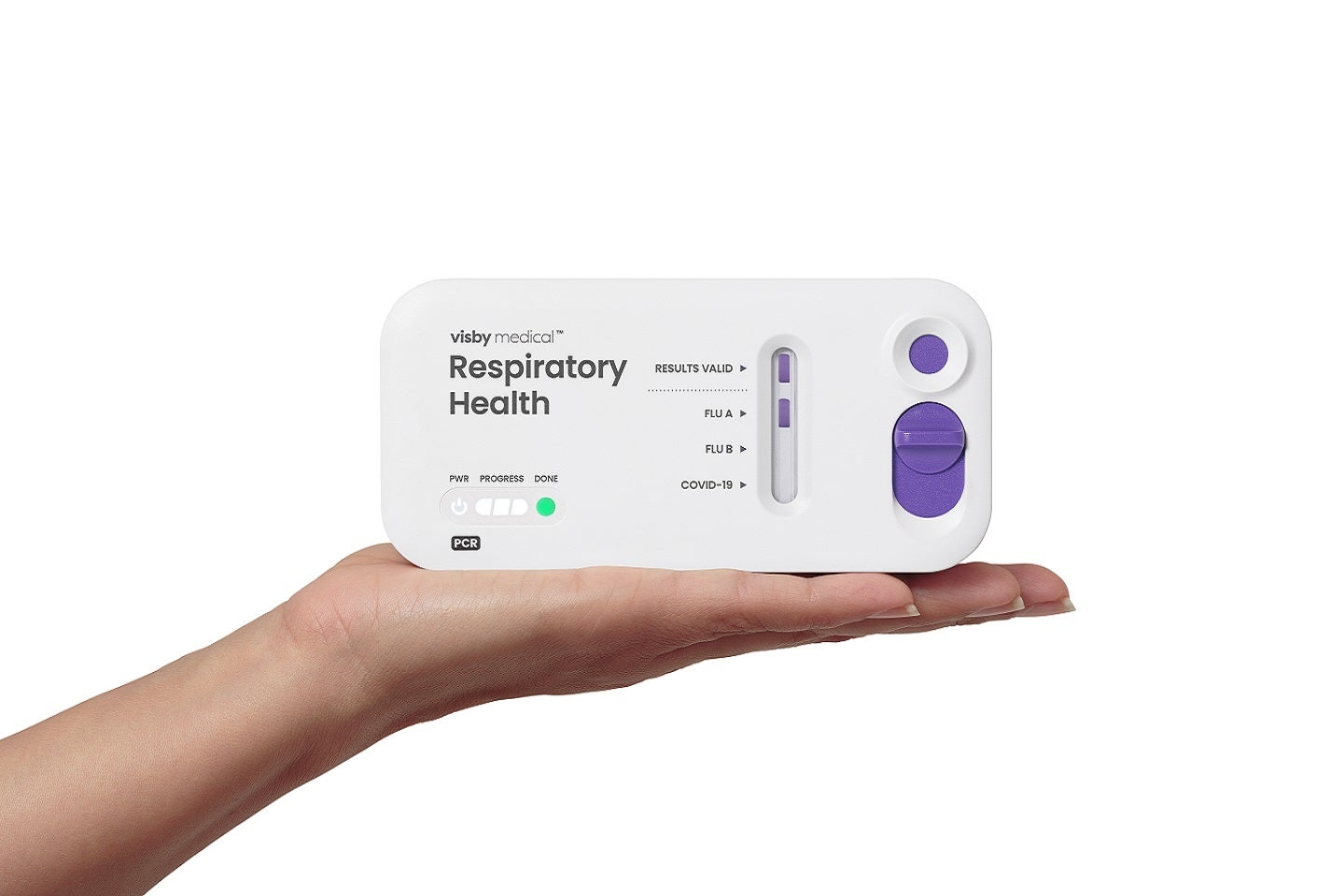
FDA grants 510(k) clearance for Visby’s respiratory infections detection test
The FDA has granted 510(k) clearance for Visby Medical’s polymerase chain reaction (PCR) test to detect respiratory infections. The post FDA grants 510(k) clearance for Visby’s respiratory infections detection test appeared first on Medical Device Network.
The post FDA grants 510(k) clearance for Visby’s respiratory infections detection test appeared first on Medical Device Network.