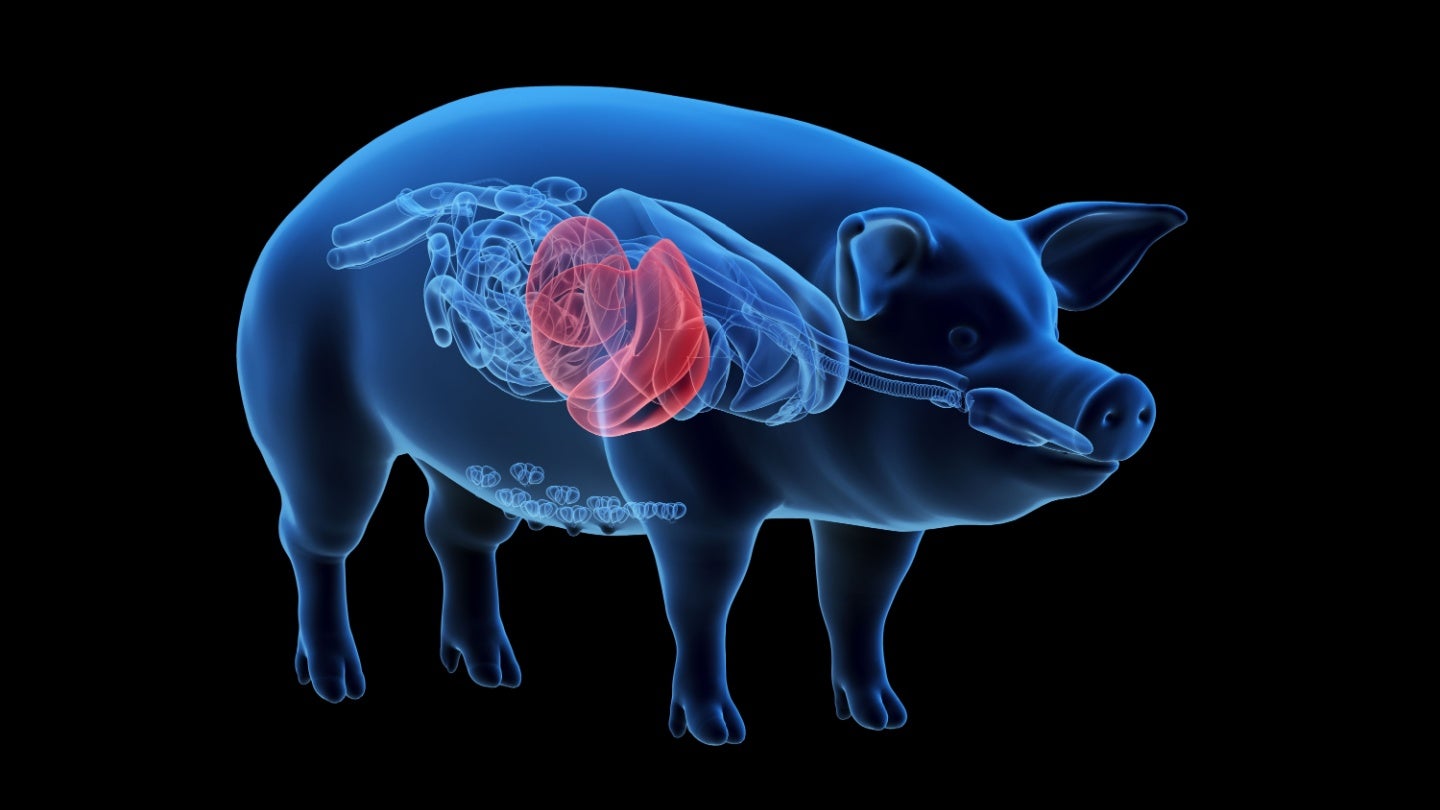
FDA clears eGenesis’ genetically engineered porcine liver for Phase I trial
eGenesis has gained US Food and Drug Administration (FDA) clearance on an Investigational New Drug (IND) application for a genetically engineered porcine (pig) liver designed for use with OrganOx’s extracorporeal liver cross-circulation (ELC) system for patients with acute-on-chronic liver failure (ACLF). The post FDA clears eGenesis’ genetically engineered porcine liver for Phase I trial appeared first on Medical Device Network.
The post FDA clears eGenesis’ genetically engineered porcine liver for Phase I trial appeared first on Medical Device Network.