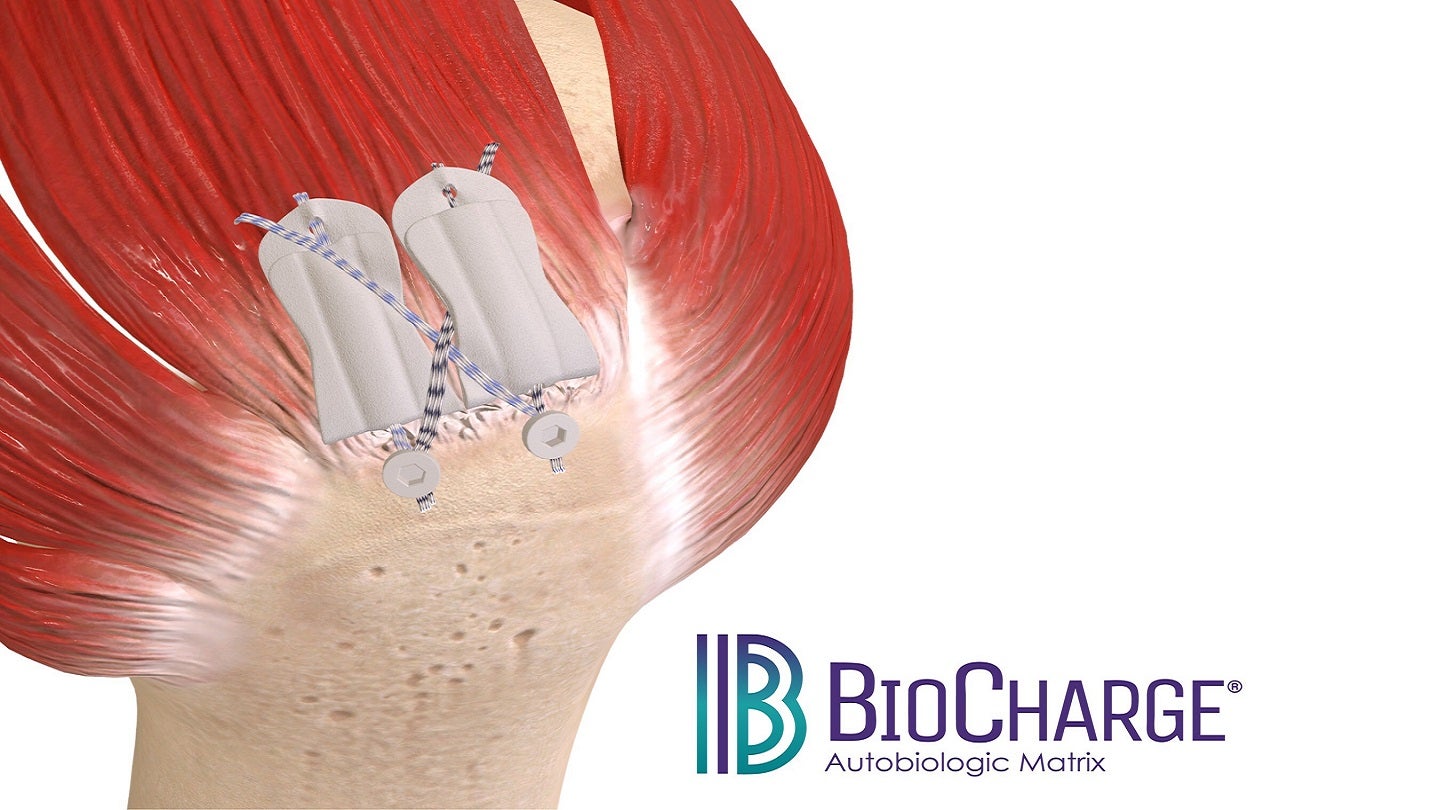
FDA clears Atreon’s bioresorbable synthetic implant for rotator cuff repair
The FDA has granted 510(k) clearance to Atreon Orthopedics’ BioCharge Autobiologic Matrix implant to enhance rotator cuff repair integrity. The post FDA clears Atreon’s bioresorbable synthetic implant for rotator cuff repair appeared first on Medical Device Network.
The post FDA clears Atreon’s bioresorbable synthetic implant for rotator cuff repair appeared first on Medical Device Network.