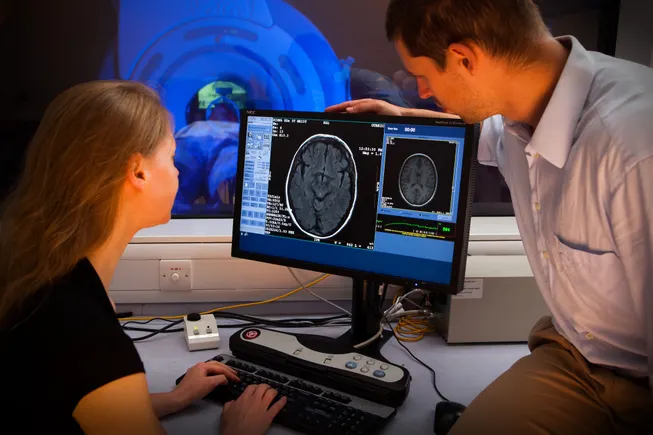
STAT+: U.K. decision blocks a pair of Alzheimer’s drugs from access through its national health service
A U.K. health agency said the Alzheimer's drugs Kisunla and Leqembi are not cost-effective, meaning they won't be offered through the NHS.

LONDON — A U.K. health agency reiterated on Thursday that the limited benefits of a pair of new Alzheimer’s drugs do not justify their high prices, meaning the medicines won’t be made available through the National Health Service.
The decision by the U.K.’s cost-effectiveness watchdog reflects how health authorities in different parts of the world have taken different approaches with the drugs, which are the first to have shown they can slow the progression of the disease but have stoked debate about just how meaningful that progress is for patients and how to weigh their accompanying risks and costs.
The drugs — Eli Lilly’s Kisunla (also known as donanemab) and Biogen and Eisai’s Leqembi (lecanemab) — won approval from U.K. regulators last year, with the Medicines and Healthcare products Regulatory Agency finding the benefits of the drugs outweighed the risks for certain patients.

















































































![The American contingent and Turkey’s autonomy goals: Paris Air Show Day 3 [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/Wednesday-Wrap.00_00_32_21.Still001.png?#)
![A look at the jets flying high above the Paris Air Show [PHOTOS]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/Rafale_02-scaled-e1750268097167.jpg?#)