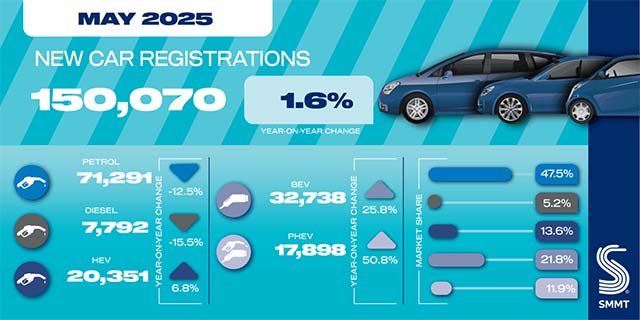
MXene and Near‐Infrared Carbon Dots Co‐Encapsulated Hydrogel Facilitates Infected Bone Defect Reconstruction
Advanced Healthcare Materials, Volume 14, Issue 13, May 16, 2025.

Ti3C2Tx with antibacterial activity and negatively charged CDs with osteogenic activity are co-encapsulated in the hydrogels for the repair of infected bone defects. The dynamic monitoring of degradation of scaffolds is realized by the NIR imaging in infected bone defect models. Even in the presence of MRSA, the nearly complete regeneration of bone defects is achieved by CD/Ti3C2Tx/GelMA hydrogels.
Abstract
Inadequate bone differentiation and intractable biofilm formation due to stubborn bacterial infection complicate infected bone defect repair. Adding harmful antibiotics into scaffolds not only promotes multidrug-resistant bacteria but also decreases bone repair effect. Furthermore, dynamic monitor of scaffolds' degradation is crucial for achieving visualized bone defect repair, however, currently reported biomaterials do not have imaging tracing capabilities. On this basis, this work develops a scaffold material with triple functionality for visualized therapy of infected bone defects: antibacterial, osteogenesis, and near-infrared (NIR) imaging capabilities. Single-layer Ti3C2Tx with broad-spectrumantibacterial activity and negatively charged carbon dots (CDs) with osteogenic activity are synthesized for infected bone defect repair. To validate antibacterial and osteogenic activities in vivo, 3D injectable hydrogels encapsulated with Ti3C2Tx and CDs (CD/Ti3C2Tx/GelMA) are constructed. NIR imaging is used to monitor the degradation process of CD/Ti3C2Tx/GelMA hydrogels in infected bone defect models, which indicated that CDs are completely released from hydrogels in about 30 days. Owing to the continuous release of Ti3C2Tx and CDs, the obtained CD/Ti3C2Tx/GelMA hydrogels can efficiently promote the repair of infected bone defects within 60 days. These findings develop a new biomaterial with great performance for visualized antibacterial and osteogenic therapy of infected bone defects.