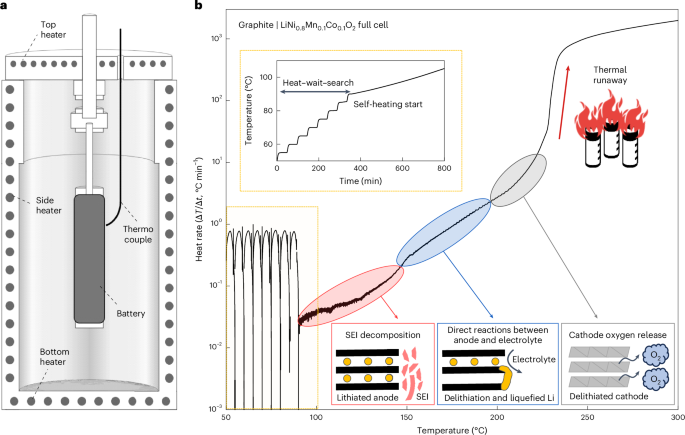
<i>Mdga2</i> deficiency leads to an aberrant activation of BDNF/TrkB signaling that underlies autism-relevant synaptic and behavioral changes in mice
by Dongdong Zhao, Yuanhui Huo, Naizhen Zheng, Xiang Zhu, Dingting Yang, Yunqiang Zhou, Shengya Wang, Yiru Jiang, Yili Wu, Yun-wu Zhang Memprin/A5/mu (MAM) domain containing glycosylphosphatidylinositol anchor 2 (MDGA2) is an excitatory synaptic suppressor and its mutations have been associated with autism spectrum disorder (ASD). However, the detailed physiological function of MDGA2 and the mechanism underlying MDGA2 deficiency-caused ASD has yet to be elucidated. Herein, we not only confirm that Mdga2 +/− mice exhibit increased excitatory synapse transmission and ASD-like behaviors, but also identify aberrant brain-derived neurotrophic factor/tyrosine kinase B (BDNF/TrkB) signaling activation in these mice. We demonstrate that MDGA2 interacts with TrkB through its memprin/A5/mu domain, thereby competing the binding of BDNF to TrkB. Both loss of MDGA2 and the ASD-associated MDGA2 V930I mutation promote the BDNF/TrkB signaling activity. Importantly, we demonstrate that inhibiting the BDNF/TrkB signaling by both small molecular compound and MDGA2-derived peptide can attenuate the increase of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor-mediated excitatory synaptic activity and social deficits in MDGA2-deficient mice. These results highlight a novel MDGA2-BDNF/TrkB-dependent mechanism underlying the synaptic function regulation, which may become a therapeutic target for ASD.
by Dongdong Zhao, Yuanhui Huo, Naizhen Zheng, Xiang Zhu, Dingting Yang, Yunqiang Zhou, Shengya Wang, Yiru Jiang, Yili Wu, Yun-wu Zhang Memprin/A5/mu (MAM) domain containing glycosylphosphatidylinositol anchor 2 (MDGA2) is an excitatory synaptic suppressor and its mutations have been associated with autism spectrum disorder (ASD). However, the detailed physiological function of MDGA2 and the mechanism underlying MDGA2 deficiency-caused ASD has yet to be elucidated. Herein, we not only confirm that Mdga2 +/− mice exhibit increased excitatory synapse transmission and ASD-like behaviors, but also identify aberrant brain-derived neurotrophic factor/tyrosine kinase B (BDNF/TrkB) signaling activation in these mice. We demonstrate that MDGA2 interacts with TrkB through its memprin/A5/mu domain, thereby competing the binding of BDNF to TrkB. Both loss of MDGA2 and the ASD-associated MDGA2 V930I mutation promote the BDNF/TrkB signaling activity. Importantly, we demonstrate that inhibiting the BDNF/TrkB signaling by both small molecular compound and MDGA2-derived peptide can attenuate the increase of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor-mediated excitatory synaptic activity and social deficits in MDGA2-deficient mice. These results highlight a novel MDGA2-BDNF/TrkB-dependent mechanism underlying the synaptic function regulation, which may become a therapeutic target for ASD.