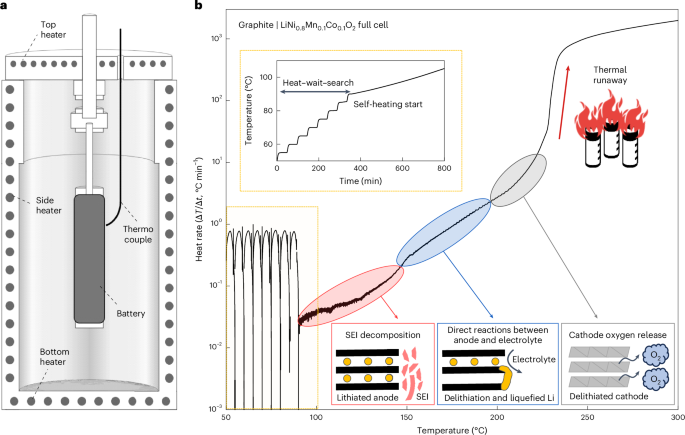
Lundbeck ends subcutaneous migraine cohort after futility review, pivots to IV formulation
Lundbeck has stopped development of a subcutaneous formulation of its migraine drug Lu AG09222. The Danish drugmaker switched its focus to intravenous delivery after seeing phase 2b data, delaying the completion of the trial in the process.