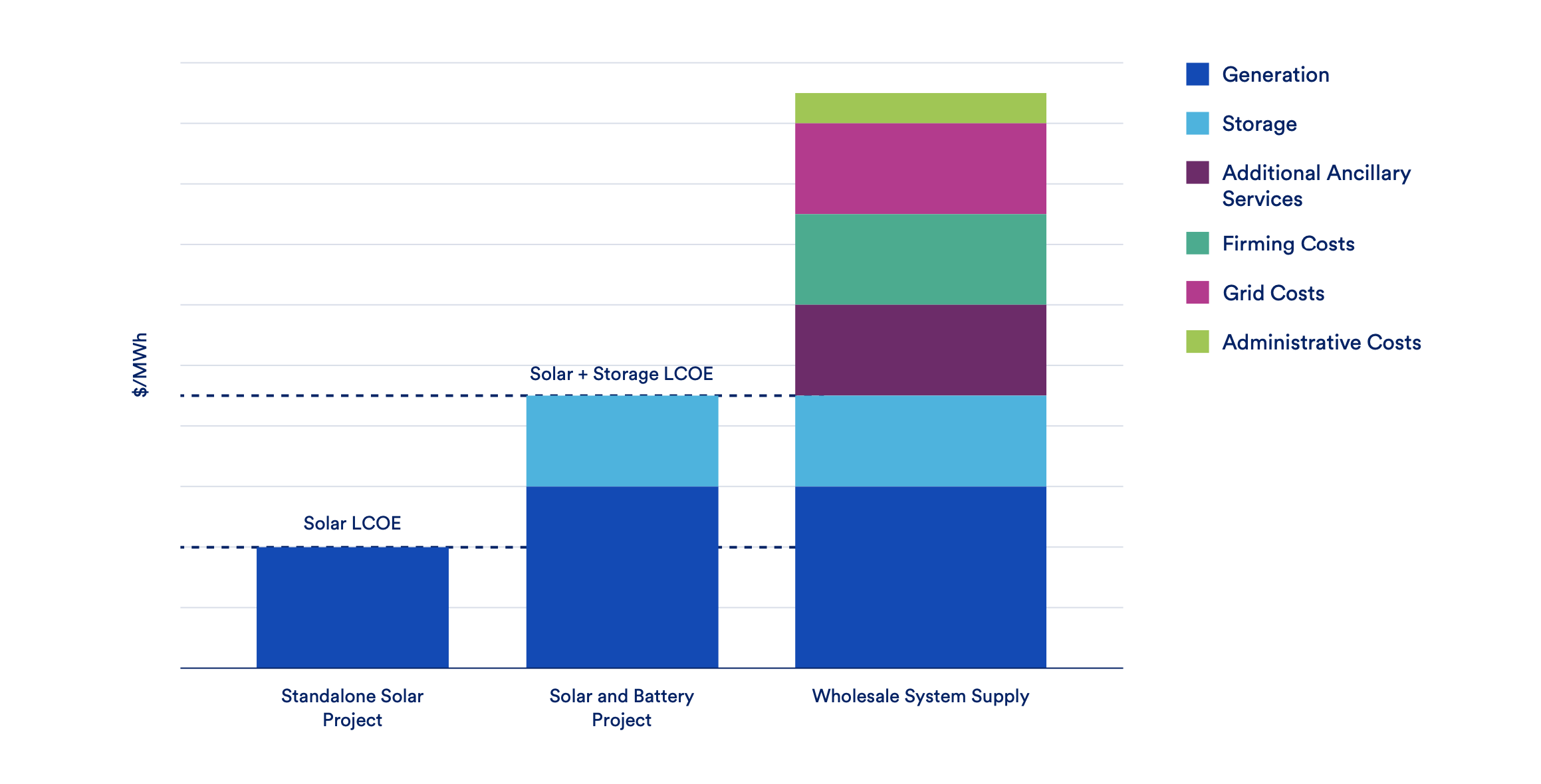
KLVFF‐Guided Molecular Scissors: a Trojan Horse Strategy for Precision Photodynamic Dissolution of Aβ Aggregates
Advanced Healthcare Materials, EarlyView.

Current Aβ-targeting strategies suffer from limited specificity, irreversible binding, and systemic toxicity. Here, a rationally engineered nanomaterial (CNPs-Ce650-K0.5) is presented that integrates dual therapeutic modalities: 1) KLVFF peptides for high-affinity recognition of Aβ monomers, competitively inhibiting nucleation, and 2) chlorin e6 (Ce6) photosensitizers for light-triggered ROS generation, enabling spatiotemporal disruption of β-sheet-rich fibrils.
Abstract
Alzheimer's disease (AD) is pathologically linked to the aggregation of amyloid-β (Aβ) peptides into neurotoxic fibrils. Despite advancements in Alzheimer's therapeutics, current strategies fail to simultaneously target amyloid-β aggregation and oxidative stress, particularly with spatiotemporal precision. To address the limitations of conventional therapies, this study develops a photo-responsive nanomaterial, conjugated polymer nanodots functionalized with chlorin e6 (Ce6) and KLVFF peptides (CNPs-Ce650-K0.5), for targeted inhibition of Aβ 1-42 fibrillization. The nanomaterial is synthesized via nanoprecipitation, integrating Ce6 for reactive oxygen species (ROS) generation and KLVFF for Aβ-specific recognition. Systematic characterization confirms its structural stability, tunable ROS production, and high biocompatibility. Fluorescence microscopy, TEM, and CD spectroscopy demonstrate that CNPs-Ce650-K0.5 synergistically inhibits Aβ 1-42 aggregation through dual mechanisms: 1) KLVFF-mediated high-affinity binding to Aβ monomers, preventing nucleation; 2) Ce6-generated ROS under light irradiation, destabilizing β-sheet structures. This work establishes CNPs-Ce650-K0.5 as a multifunctional platform for precise Aβ modulation, offering a promising strategy for AD therapeutics with spatiotemporal controllability and minimal off-target effects.