This site uses cookies. By continuing to browse the site you are agreeing to our use of cookies.
All
Agriculture and Farming
Agriculture and Food News -- ScienceDaily
CropLife
Farming Today
Modern Farmer
National Sustainable Agriculture Coalition
Trump’s Conflicting Messages on Workplace Rai...
Jun 18, 2025 0
Los menonitas de México que se asociaron con ...
Jun 17, 2025 0
Trump’s Conflicting Messages on Workplace Rai...
Jun 18, 2025 0
Los menonitas de México que se asociaron con ...
Jun 17, 2025 0
El problema con las garrapatas está empeorand...
Jun 17, 2025 0
The Tick Situation Is Getting Worse
Jun 16, 2025 0
All
Autoblog
Autocar RSS Feed
Automotive News Breaking News Feed
Automotive World
Autos
Electric Cars Report
Jalopnik
Automotive News | AM-online
Speedhunters
The Truth About Cars
Tata Technologies has been selected as a stra...
Jun 19, 2025 0
Stellantis extends stop-drive action in Europ...
Jun 19, 2025 0
Helm.ai announces Level 3 urban perception sy...
Jun 19, 2025 0
Wallbox and PowerGo announce collaboration to...
Jun 19, 2025 0
All
All Stories
All Stories
BioPharma Dive - Latest News
Breaking World Pharma News
Drugs.com - Clinical Trials
Drugs.com - FDA MedWatch Alerts
Drugs.com - New Drug Approvals
Drugs.com - Pharma Industry News
FDA Press Releases RSS Feed
Federal Register: Food and Drug Administration
News and press releases
Pharmaceuticals news FT.com
PharmaTimes World News
Stat
What's new
Data access to medicinal products for human u...
Jun 19, 2025 0
Improving T cell responses to vaccines
Jun 15, 2025 0
Fermenting legume pulses boosts their antidia...
Jun 15, 2025 0
Ultra-selective aptamers give viruses a taste...
Jun 15, 2025 0
Data access to medicinal products for human u...
Jun 19, 2025 0
Products Management Services (PMS) - Implemen...
Jun 19, 2025 0
All
Breaking DefenseFull RSS Feed – Breaking Defense
DefenceTalk
Defense One - All Content
Military Space News
NATO Latest News
The Aviationist
War is Boring
War on the Rocks
NATO Deputy Secretary General attends the int...
Jun 19, 2025 0
Chair of the NATO Military Committee visits T...
Jun 18, 2025 0
NATO Secretary General attends G7 Summit, wel...
Jun 17, 2025 0
Participation by the NATO Secretary General i...
Jun 16, 2025 0
All
Advanced Energy Materials
CleanTechnica
Energy | FT
Energy | The Guardian
EnergyTrend
Nature Energy
NYT > Energy & Environment
PV-Tech
RSC - Energy Environ. Sci. latest articles
Utility Dive - Latest News
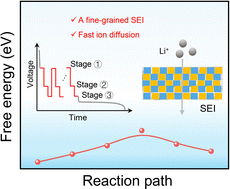
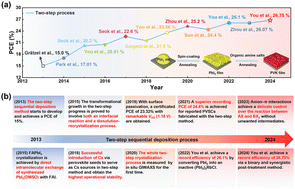
The Origin of Improved Performance in Boron‐A...
Jun 19, 2025 0
Tuning Cation (Dis)Order in Cr‐Based Li‐Exces...
Jun 19, 2025 0
An Additive‐Assisted Hydrolysis‐Blocking Rout...
Jun 19, 2025 0
Enhancing Long‐Cycle Performance of Zinc Powd...
Jun 19, 2025 0
Local energy initiatives
Jun 18, 2025 0
Stacked for transparency
Jun 17, 2025 0
Multi-Physics Mechanisms and Regulation of Pe...
Jun 19, 2025 0
- Contact
- LIVE TV
- Agriculture
- Automotive
- Beauty
-
Biopharma
- All
- All Stories
- All Stories
- BioPharma Dive - Latest News
- Breaking World Pharma News
- Drugs.com - Clinical Trials
- Drugs.com - FDA MedWatch Alerts
- Drugs.com - New Drug Approvals
- Drugs.com - Pharma Industry News
- FDA Press Releases RSS Feed
- Federal Register: Food and Drug Administration
- News and press releases
- Pharmaceuticals news FT.com
- PharmaTimes World News
- Stat
- What's new
- Defense
- Energy & Water
- Fashion
- Food & Beverage
- Healthcare
- Legal
- Manufacturing
- Luxury
- Medical Devices
- Mining
- Real Estate
- Retail
- Science Journals
- Transport & Logistics
- Travel & Hospitality

















































































![Four big takeaways to wrap up the Paris Air Show [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2023/06/FCAS-scaled-e1685718848105.jpg?#)
![The sights of Paris Air Show, one last time: Day 4 [Photos]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/20250617-helenedelacoste-Paris-Air-Show-037-scaled-e1750357690820.jpg?#)