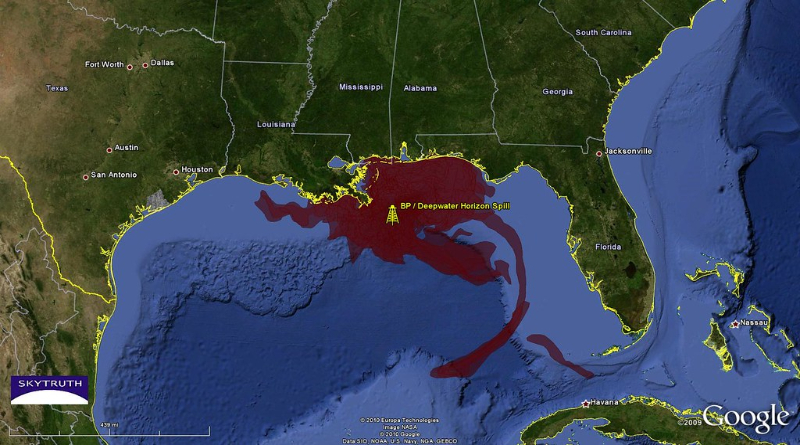
STAT+: FDA probes deaths of boys taking Sarepta Duchenne drug
And other biotech news stories brought to you by The Readout.

Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Good morning, I hope you’re able to stay cool wherever you are. Let’s get into the news today.
The need-to-know this morning
- Viking Therapeutics announced the start of Phase 3 studies involving its injectable, GLP-1/GIP-targeted obesity drug, called VK2735.
- Gilead Sciences and Kymera Therapeutics announced a partnership and licensing agreement to develop a new class of cancer drugs that eliminates a protein called CDK2 from cells — a contributor to tumor growth.
- Biogen is advancing a next-generation treatment for spinal muscular atrophy into a Phase 3 clinical trial.
As FDA center director exits, a new hire is announced
A day after the top drug regulator at the Food and Drug Administration announced she was leaving her post, a new deputy director of the office she led, the Center for Drug Evaluation and Research, was named yesterday.