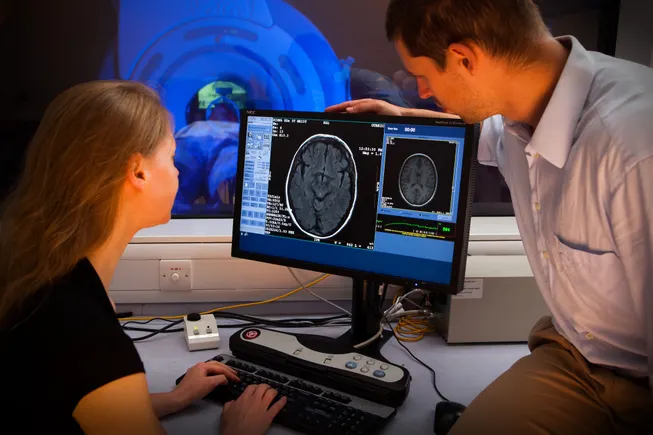
STAT+: As AI device market grows, FDA’s accounting goes silent
As the market for AI enabled medical devices grows, experts say there is less transparency around device approvals.

In December, as the Food and Drug Administration was finalizing an avalanche of last-minute regulatory guidelines before President Trump’s inauguration, it quietly passed a major milestone. A regularly-updated list from its device center showed the FDA had authorized more than 1,000 devices enabled by artificial intelligence and machine learning, mirroring the rapid growth of the technology in health care.
In the six months since, as the federal government has moved to deregulate the field of AI, the FDA’s list of AI/ML device authorizations has gone untouched.
Commissioner Marty Makary has moved quickly to launch and publicize internal AI tools for the agency with the goal of reducing scientific review time. But he hasn’t been as vocal about its plans for regulating products that use AI, including the hot-button issue of generative AI.





















































































![Four big takeaways to wrap up the Paris Air Show [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2023/06/FCAS-scaled-e1685718848105.jpg?#)
![The sights of Paris Air Show, one last time: Day 4 [Photos]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/20250617-helenedelacoste-Paris-Air-Show-037-scaled-e1750357690820.jpg?#)