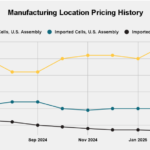
Human medicines European public assessment report (EPAR): Hyrimoz, adalimumab, Date of authorisation: 26/07/2018, Revision: 16, Status: Authorised
Human medicines European public assessment report (EPAR): Hyrimoz, adalimumab, Date of authorisation: 26/07/2018, Revision: 16, Status: Authorised






















































































































![Why Did Tesla Stock [TSLA] Rise Again?](https://cleantechnica.com/wp-content/uploads/2025/04/Screenshot-2025-04-10-at-3.31.36 AM.png)