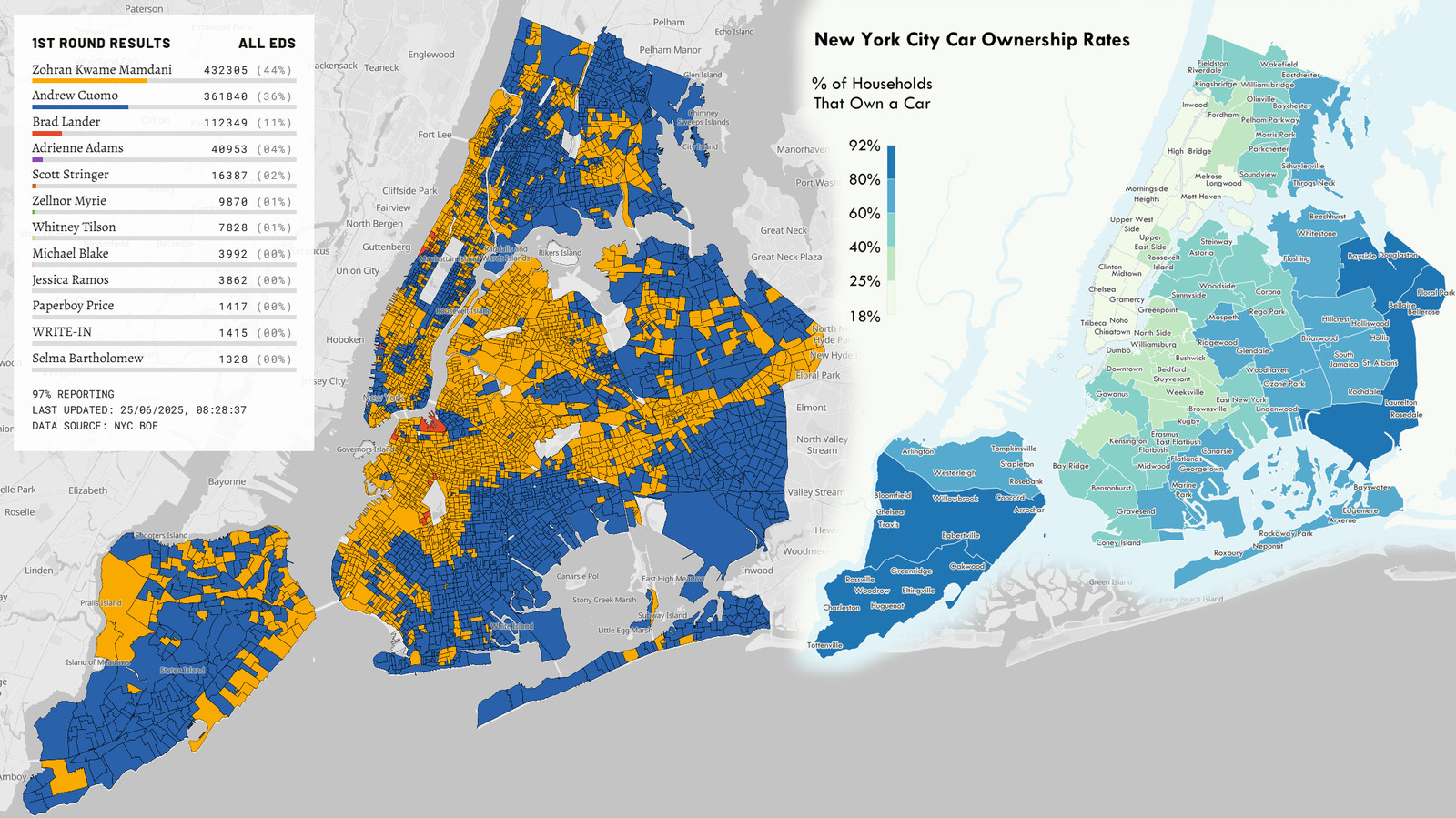
FDA investigates patient deaths after treatment with Sarepta's Duchenne gene therapy Elevidys
In one of the first major tests of the new FDA leadership’s regulatory philosophy toward gene therapies for rare diseases, the agency is investigating two Duchenne muscular dystrophy patient deaths following treatment with Sarepta Therapeutics’ Elevidys.