FDA follows EMA in limiting use of Valneva shot
Reports of severe adverse events in some people who received the chikungunya vaccine have spurred regulators in Europe and now the U.S. to suspend use in older adults pending an investigation.
Reports of severe adverse events in some people who received the chikungunya vaccine have spurred regulators in Europe and now the U.S. to suspend use in older adults pending an investigation.















































































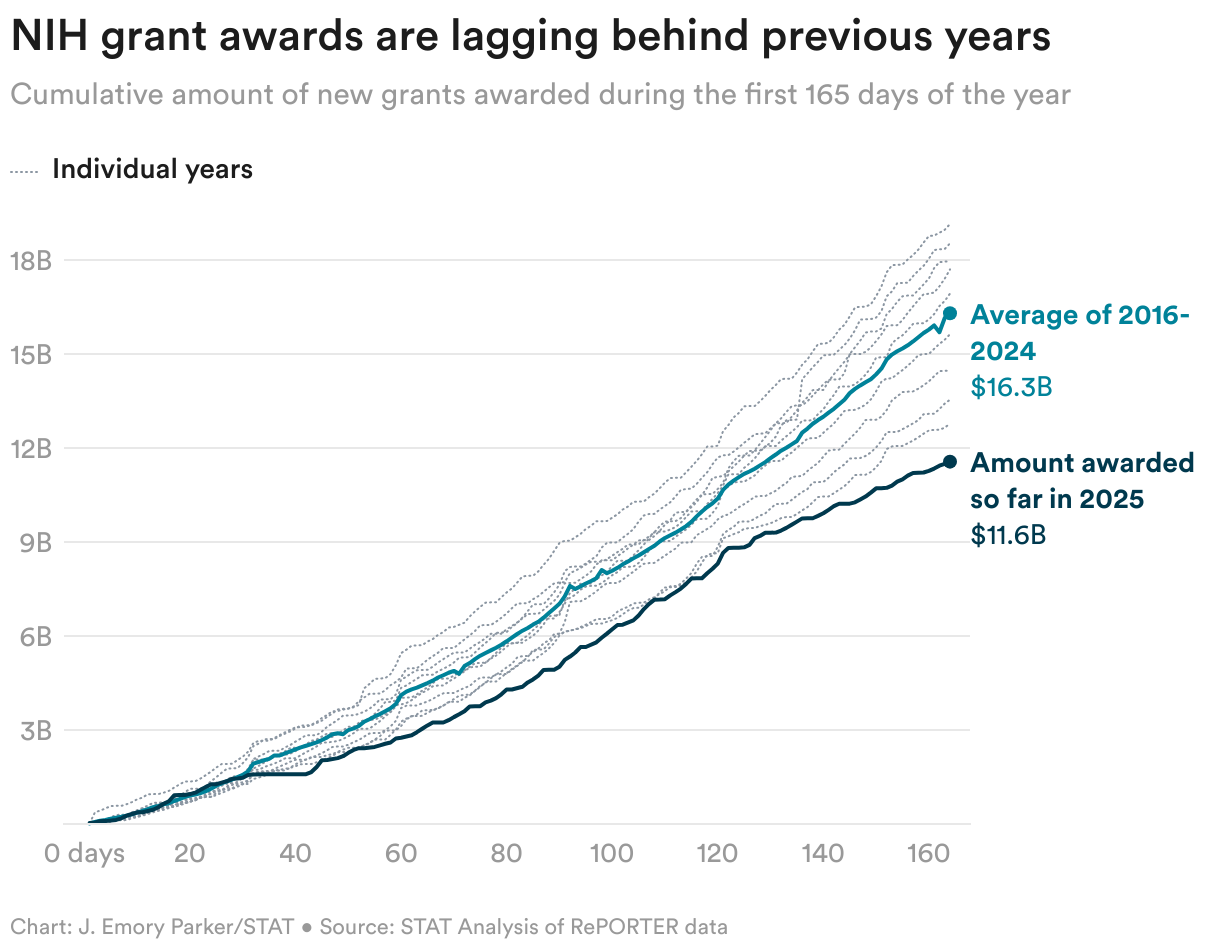




























































![[Podcast] Behind the Breakthroughs: How Almac Powers Clinical Trial Success with Care](https://imgproxy.divecdn.com/5lAJkli_KcGt1FSsw4EaegjgP76IHREqYEWbhNBJOXw/g:ce/rs:fit:770:435/Z3M6Ly9kaXZlc2l0ZS1zdG9yYWdlL2RpdmVpbWFnZS9CaW9QaGFybWFEaXZlXzEzNDZfeF83MjlfQXJ0d29yay5qcGc=.webp)

