FDA clears Miach Orthopaedics ACL implant for expanded use
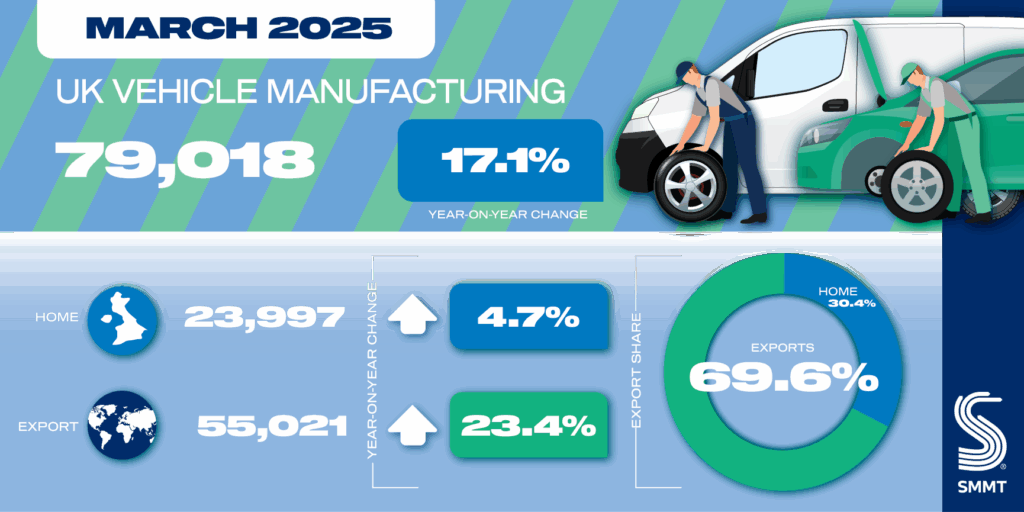
The US Food and Drug Administration (FDA) has cleared Miach Orthopaedics Bridge-Enhanced ACL Restoration (BEAR) implant for expanded use in treating complete and partial anterior cruciate ligament (ACL) tears in children of any age. The post FDA clears Miach Orthopaedics ACL implant for expanded use appeared first on Medical Device Network.

The post FDA clears Miach Orthopaedics ACL implant for expanded use appeared first on Medical Device Network.