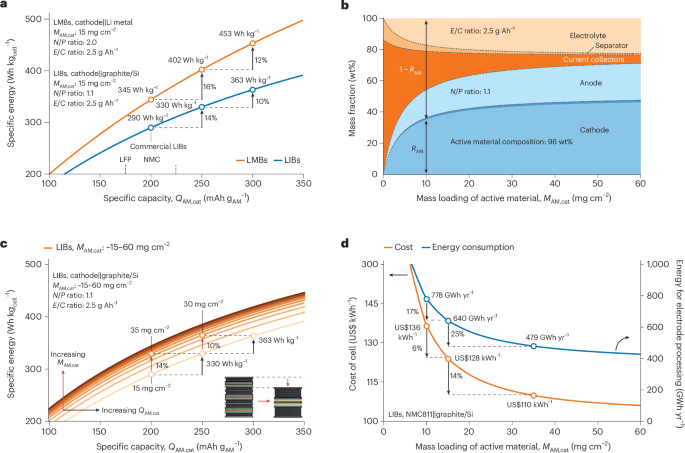
FDA Approves First Rapid-Acting Insulin Biosimilar Product for Treatment of Diabetes
FDA approved the first rapid-acting biosimilar insulin product, Merilog (insulin-aspart-szij), a biosimilar to its reference product Novolog (insulin aspart), to improve glycemic control in adults and children with diabetes mellitus.