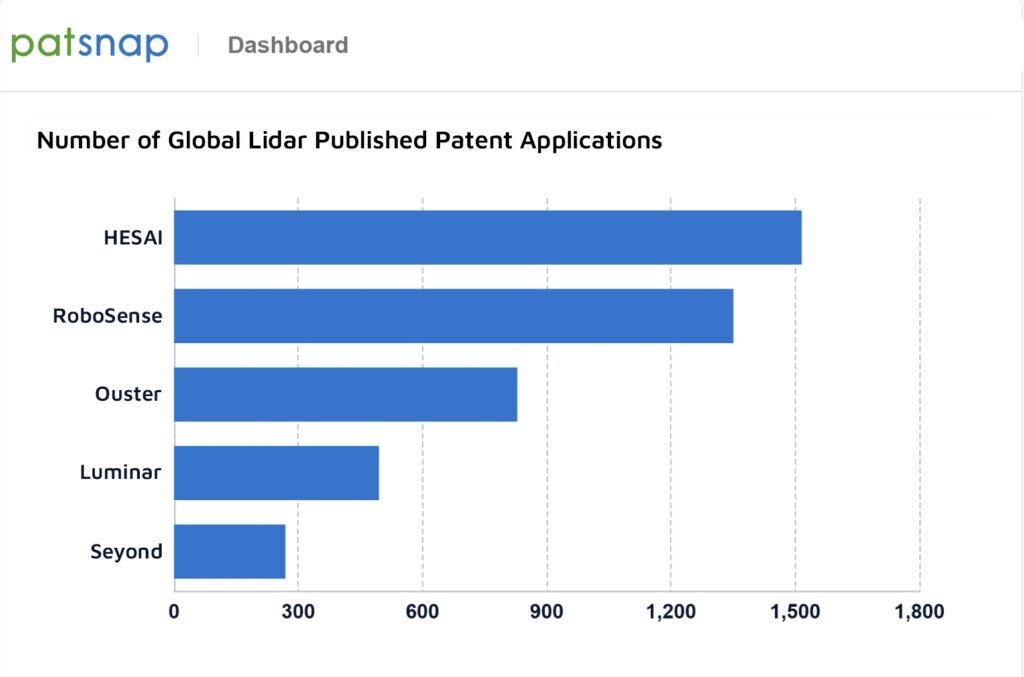
Modulation of NH2‐UiO‐66 Based MOFs for Gas Phase CO2 Photocatalytic Reduction
Advanced Energy Materials, EarlyView.

This study addresses the dual challenges of the greenhouse effect and energy crisis through the direct capture and conversion of CO2 using Metal-Organic Frameworks (MOFs). By synthesizing defective NH2-UiO-66 MOFs and combining them with TiO2 and gold nanoparticles, a ternary photocatalyst that significantly enhances CO2 adsorption and CH4 production, achieving a six-fold increase in photocatalytic activity compared to a TiO2/Au reference catalyst is developed.
Abstract
To solve both the greenhouse effect and energy crisis issues, direct capture and conversion of CO2 appears as a promising contribution. Artificial photosynthesis is an encouraging and very attractive complementary way to convert CO2 into high-value-added chemicals. Nevertheless, efficient CO2 conversion remains challenging, mainly due to the poor CO2 adsorption, the high activation energy required to break C═O bonds, and the slow dynamics of charge carriers in light-harvesting materials. In recent years, some Metal-organic frameworks (MOFs) have emerged in the photocatalysis field, especially due to their huge CO2 adsorption capability and light-harvesting properties behavior. In this study, a series of defective NH2-UiO-66 MOF materials, using formic acid (FA) is synthesized as a modulating agent. Then, a series of binary (MOF/TiO2) catalysts is obtained by varying the defective MOFs content, followed by further deposition of gold (Au) nanoparticles (NPs) to result in three-component MOF/TiO2/Au composites. These composites are assessed for gas-phase CO2 photoreduction in the presence of water vapor as the only reducing agent. This hybrid ternary photocatalyst not only increases CO2 adsorption but also promotes CH4 formation. Among these resulting MOF/TiO2/Au samples, the optimized photocatalytic activity results in a mean production rate of 17.2 µmol.h−1.g−1 catalyst of CH4, which is 6-fold higher than the one observed for the reference TiO2/Au photocatalyst.