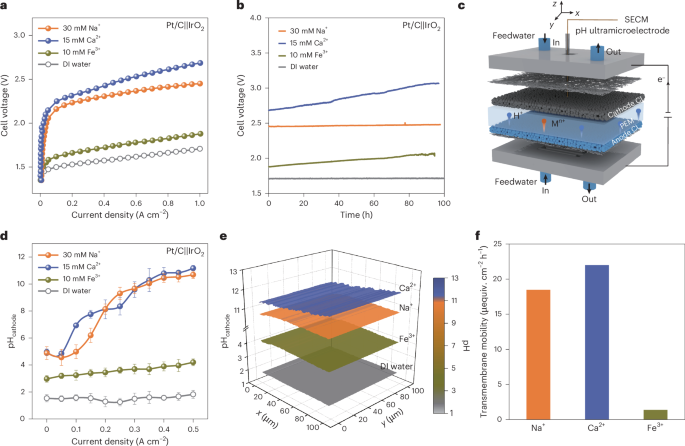
Enhancing Specific Energy and Cycling Stability of High‐Temperature Na‐ZnCl2 Batteries with Foam‐Based Electrodes
Advanced Energy Materials, EarlyView.

Replacing nickel with zinc in high-temperature sodium-zinc chloride (Na-ZnCl₂) batteries makes them more cost-effective and sustainable. Foam-based Zn/NaCl electrodes enable substantially increased metal utilization compared to granule-based electrodes (66% vs 30%). They also enhance cycle stability and significantly boost specific energy (336 Wh kg⁻¹ vs 231 Wh kg⁻¹ at 15 mA cm⁻2).
Abstract
Sodium–zinc chloride (Na-ZnCl₂) batteries offer a sustainable alternative to sodium–nickel chloride (Na-NiCl₂) batteries but face challenges with low specific energy and cycle life. This study evaluates two electrode designs: conventional particle-based Zn/NaCl granules and newly developed foam-based Zn/NaCl electrodes. Particle-based electrodes, with 30% Zn utilization, cycled in tubular cells with a mass loading of 1.13 g cm−2 and an areal capacity of 150 mAh cm−2, achieve a specific energy of 231 Wh kg⁻¹ on electrode composite level at 15 mA cm⁻2 but suffer from degradation in voltage efficiency due to Zn agglomeration. To address this, foam-based Zn/NaCl electrodes are developed, enhancing Zn utilization to 66%. Cycled in planar Na-ZnCl₂ cells, these foam-based electrodes achieve over 200 mAh cm−2 areal capacity at a mass loading of 1.04 g cm−2, providing a specific energy of 336 Wh kg⁻¹ at 15 mA cm⁻2 with stable voltage profiles. The foam-based design stabilizes the electrode microstructure, delivering a high cumulative discharge capacity of 5.4 Ah cm⁻2 with stable voltage efficiency. These results represent the highest mass loadings and areal capacities reported for sodium metal chloride cells to date, demonstrating their potential for enabling cost-effective Na-ZnCl₂ batteries for stationary energy storage applications.
























































![[Podcast] Trial Trailblazers: Behind clinical breakthroughs](https://imgproxy.divecdn.com/cr_gUY8HHfHKWVfM5mEkCyXB9OzVqKd0L_Zq5ZJQSoM/g:ce/rs:fit:770:435/Z3M6Ly9kaXZlc2l0ZS1zdG9yYWdlL2RpdmVpbWFnZS9CaW9QaGFybWFEaXZlX1BvZGNhc3RfY292ZXIxMzQ2eDcyOV9SMi5wbmc=.webp)