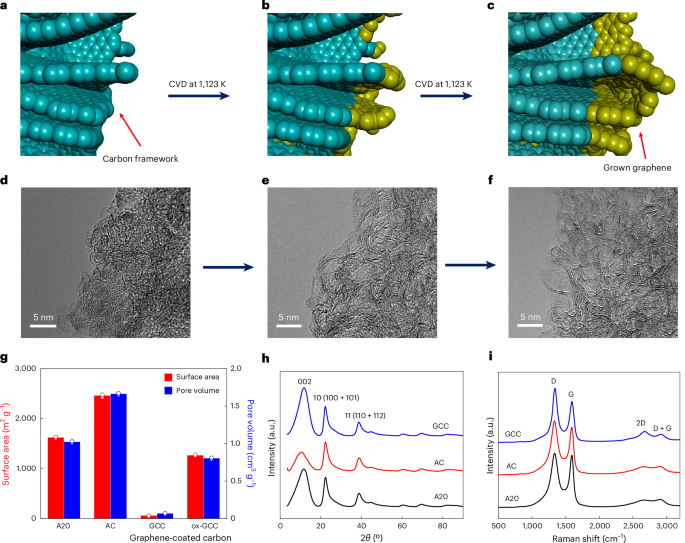
Disruption of HaVipR1 confers Vip3Aa resistance in the moth crop pest <i>Helicoverpa armigera</i>
by Andreas Bachler, Amanda Padovan, Craig J. Anderson, Yiyun Wei, Yidong Wu, Stephen Pearce, Sharon Downes, Bill James, Ashley E. Tessnow, Gregory A. Sword, Michelle Williams, Wee Tek Tay, Karl H. J. Gordon, Tom K. Walsh The global reliance on Bacillus thuringiensis (Bt) proteins for controlling lepidopteran pests in cotton, corn, and soybean crops underscores the critical need to understand resistance mechanisms. Vip3Aa, one of the most widely deployed and currently effective Bt proteins in genetically modified crops, plays a pivotal role in pest management. This study investigates the molecular basis of Vip3Aa resistance in Australian Helicoverpa armigera through genetic crosses, and integrated genomic and transcriptomic analyses. We identified a previously uncharacterized gene, LOC110373801 (designated HaVipR1), as potentially important in Vip3Aa resistance in two field-derived resistant lines. Functional validation using CRISPR/Cas9 knockout in susceptible lines confirmed the gene’s role in conferring high-level resistance to Vip3Aa. Despite extensive laboratory selection of Vip3Aa-resistant colonies in Lepidoptera, the biochemical mechanisms underlying resistance have remained elusive. Our research identifies HaVipR1 as a potential contributor to resistance, adding to our understanding of how insects may develop resistance to this important Bt protein. The identification of HaVipR1 contributes to our understanding of potential resistance mechanisms and may inform future resistance management strategies. Future work should explore the biochemical pathways influenced by HaVipR1 and assess its interactions with other resistance mechanisms. The approach utilized here underscores the value of field-derived resistant lines for understanding resistance in agricultural pests and highlights the need for targeted approaches to manage resistance sustainably.
by Andreas Bachler, Amanda Padovan, Craig J. Anderson, Yiyun Wei, Yidong Wu, Stephen Pearce, Sharon Downes, Bill James, Ashley E. Tessnow, Gregory A. Sword, Michelle Williams, Wee Tek Tay, Karl H. J. Gordon, Tom K. Walsh The global reliance on Bacillus thuringiensis (Bt) proteins for controlling lepidopteran pests in cotton, corn, and soybean crops underscores the critical need to understand resistance mechanisms. Vip3Aa, one of the most widely deployed and currently effective Bt proteins in genetically modified crops, plays a pivotal role in pest management. This study investigates the molecular basis of Vip3Aa resistance in Australian Helicoverpa armigera through genetic crosses, and integrated genomic and transcriptomic analyses. We identified a previously uncharacterized gene, LOC110373801 (designated HaVipR1), as potentially important in Vip3Aa resistance in two field-derived resistant lines. Functional validation using CRISPR/Cas9 knockout in susceptible lines confirmed the gene’s role in conferring high-level resistance to Vip3Aa. Despite extensive laboratory selection of Vip3Aa-resistant colonies in Lepidoptera, the biochemical mechanisms underlying resistance have remained elusive. Our research identifies HaVipR1 as a potential contributor to resistance, adding to our understanding of how insects may develop resistance to this important Bt protein. The identification of HaVipR1 contributes to our understanding of potential resistance mechanisms and may inform future resistance management strategies. Future work should explore the biochemical pathways influenced by HaVipR1 and assess its interactions with other resistance mechanisms. The approach utilized here underscores the value of field-derived resistant lines for understanding resistance in agricultural pests and highlights the need for targeted approaches to manage resistance sustainably.