Breaking the Upper Limit of Substitution Concentration in Li Argyrodite Solid Electrolytes Using a Single‐Solvent‐Mediated Approach
Advanced Energy Materials, Volume 15, Issue 20, May 27, 2025.

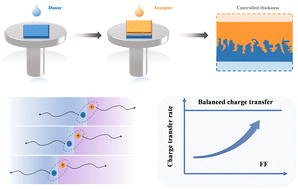
The upper limit of Si substitution level in the Li argyrodite solid electrolyte is raised using a solvent-mediated approach. Smaller particles synthesized through this method exhibit a higher surface-area-to-volume ratio, allowing for the incorporation of more Si and Li within larger amounts of interfacial space-charge layers, compared to the larger particles produced by solid-state ball milling.
Abstract
Although raising the substitution concentration of aliovalent cations in Li argyrodite solid electrolytes could boost solid-state battery performance, surpassing the known substitution limit has not been attempted. In this study, the upper substitution limit of a Li6+xP1−xSixS5Br solid electrolyte is increased using a single-solvent-mediated approach. The limit attained through this method is ≈40%, whereas that achieved through solid-state ball milling is ≈30%. This result is validated by monitoring variations in the interplanar distance, Raman shift, and ionic conductivity with respect to the substitution level. The ionic conductivity of Li6.4P0.6Si0.4S5Br is as high as ≈3.1 mS cm−1, exceeding that accomplished through ball milling. The enhanced limit is ascribed to the reduced particle size, which leads to an increased surface-area-to-volume ratio of the particles. This interpretation is supported by a theoretical formalism developed based on substituent accumulation within the space-charge layers, which predicts how the technical limit depends on the surface-volume fraction. A Li// Li6.4P0.6Si0.4S5Br//Li symmetric cell demonstrates excellent Li plating and stripping over extended cycling. A full cell incorporating Li6.4P0.6Si0.4S5Br retains ≈67% (96 mAh g−1) of its initial capacity (143 mAh g−1) after 50 cycles at 0.2 C, and delivers 76 mAh g−1 at 1 C.





























![New York City Officially Has Mechanical Garbage Trucks Now [Update]](https://www.jalopnik.com/img/gallery/new-york-city-officially-has-mechanical-garbage-trucks-now/l-intro-1749064637.jpg?#)