This site uses cookies. By continuing to browse the site you are agreeing to our use of cookies.
All
Agriculture and Farming
Agriculture and Food News -- ScienceDaily
CropLife
Farming Today
Modern Farmer
National Sustainable Agriculture Coalition
Adhesives And Sealants Market to Redefine the...
Jun 5, 2025 0
Next Big Thing? Textile Chemicals Market is o...
Jun 5, 2025 0
Air Compressor Market to Redefine the Future ...
Jun 5, 2025 0
It’s Not Just Poor Rains Causing Drought. The...
Jun 4, 2025 0
14 Million Honeybees Escape After a Truck Rol...
Jun 3, 2025 0
A Peach and Apple Farmer’s Uphill Quest to Fe...
Jun 2, 2025 0
Which Cooking Oil Is Best for the Planet?
Jun 2, 2025 0
All
Autoblog
Autocar RSS Feed
Automotive News Breaking News Feed
Automotive World
Autos
Electric Cars Report
Jalopnik
Automotive News | AM-online
Speedhunters
The Truth About Cars
Comau to participate in CWIEME 2025 with adva...
Jun 5, 2025 0
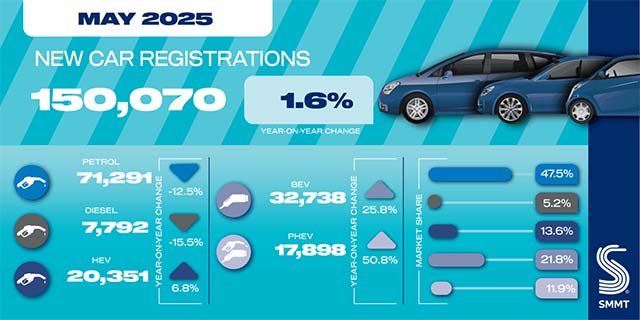
SMMT: UK new car market returns to growth thr...
Jun 5, 2025 0
Executive board for Continental spin out Aumo...
Jun 5, 2025 0
Infineon collaborates with Typhoon on real-ti...
Jun 5, 2025 0
All
All Stories
All Stories
BioPharma Dive - Latest News
Breaking World Pharma News
Drugs.com - Clinical Trials
Drugs.com - FDA MedWatch Alerts
Drugs.com - New Drug Approvals
Drugs.com - Pharma Industry News
FDA Press Releases RSS Feed
Federal Register: Food and Drug Administration
News and press releases
Pharmaceuticals news FT.com
PharmaTimes World News
Stat
What's new
On-boarding of users to Substance, Product, O...
Jun 6, 2025 0
Executive Steering Group on Shortages and Saf...
Jun 6, 2025 0
Potential new treatment for Alzheimer's ...
Jun 1, 2025 0
Researchers identify drug candidate for diffi...
Jun 1, 2025 0
DeepSeek-R1 offers promising potential to acc...
Jun 1, 2025 0
On-boarding of users to Substance, Product, O...
Jun 6, 2025 0
Executive Steering Group on Shortages and Saf...
Jun 6, 2025 0
All
Breaking DefenseFull RSS Feed – Breaking Defense
DefenceTalk
Defense One - All Content
Military Space News
NATO Latest News
The Aviationist
War is Boring
War on the Rocks
NATO Defence Ministers agree new capability t...
Jun 5, 2025 0
NATO Allies enhance cooperation in the air
Jun 5, 2025 0
NATO announces nomination of Lieutenant Gener...
Jun 5, 2025 0
NATO Secretary General to visit the United Ki...
Jun 5, 2025 0
All
Advanced Energy Materials
CleanTechnica
Energy | FT
Energy | The Guardian
EnergyTrend
Nature Energy
NYT > Energy & Environment
PV-Tech
RSC - Energy Environ. Sci. latest articles
Utility Dive - Latest News
Interior Morphology and Pore Structure in Hig...
Jun 5, 2025 0
Sulfonic Surfactant Promises Uniform Wide‐Ban...
Jun 5, 2025 0
Recycling of End‐of‐Life Lithium–Sulfur Batte...
Jun 5, 2025 0
A Novel Kinetically‐Driven Approach to Formin...
Jun 5, 2025 0
- Contact
- LIVE TV
- Agriculture
- Automotive
- Beauty
-
Biopharma
- All
- All Stories
- All Stories
- BioPharma Dive - Latest News
- Breaking World Pharma News
- Drugs.com - Clinical Trials
- Drugs.com - FDA MedWatch Alerts
- Drugs.com - New Drug Approvals
- Drugs.com - Pharma Industry News
- FDA Press Releases RSS Feed
- Federal Register: Food and Drug Administration
- News and press releases
- Pharmaceuticals news FT.com
- PharmaTimes World News
- Stat
- What's new
- Defense
- Energy & Water
- Fashion
- Food & Beverage
- Healthcare
- Legal
- Manufacturing
- Luxury
- Medical Devices
- Mining
- Real Estate
- Retail
- Science Journals
- Transport & Logistics
- Travel & Hospitality