STAT+: Verve gene-editing therapy lowers cholesterol without serious side effects in early study
Verve Therapeutics said Monday that initial data show that its investigational gene-editing therapy lowered cholesterol without inducing serious side effects.

Verve Therapeutics said initial data show that its investigational gene-editing therapy lowered cholesterol without inducing serious side effects, a positive step for the company after it paused development of an earlier treatment due to safety concerns.
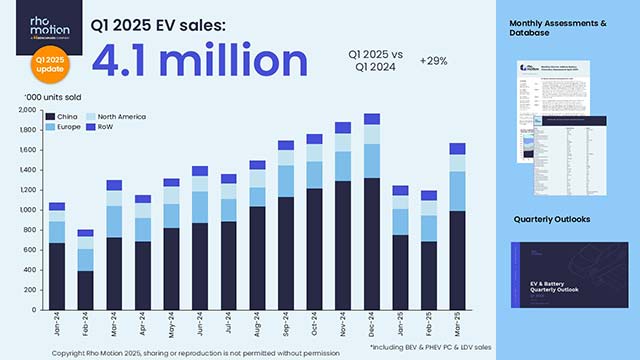
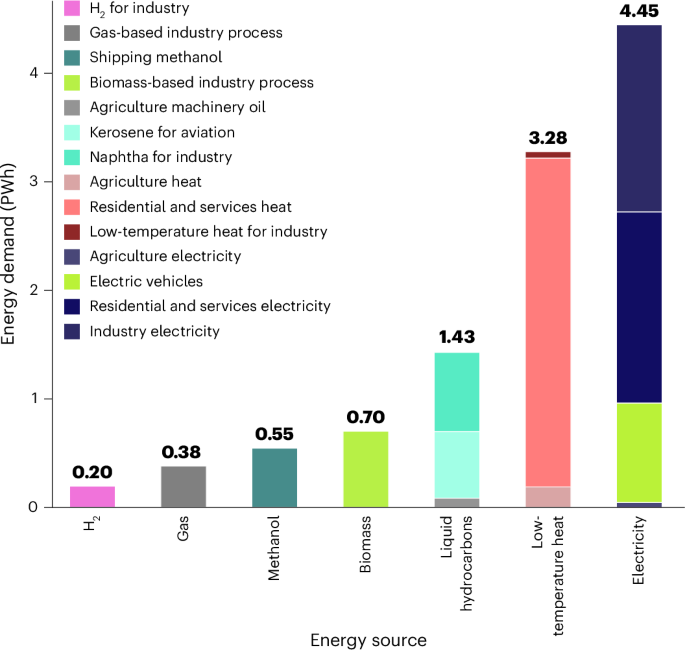
The early data from an ongoing Phase 1 study show that a single infusion of the therapy, called Verve-102, led to greater decreases in “bad” LDL cholesterol with higher doses, according to an announcement Monday. Among the four participants who received the highest dose of 0.6 mg/kg, they experienced an average 53% reduction in cholesterol.
Verve-102 is designed to turn off the PCSK9 gene in the liver that’s involved in the regulation of cholesterol. Last year, Verve paused testing of an earlier version of the therapy, Verve-101, after a patient experienced elevated liver enzymes and low platelet levels. The side effects were not thought to be related to the gene-editing component, but to the external coating of the therapy, called a lipid nanoparticle, or LNP. The company says that Verve-102 uses a different LNP developed on its own that it thinks will make the treatment safer.