STAT+: French trial sponsors are urged to bolster clinical trial transparency
A French government working group recommended a sweeping plan to publicize study data and issue guidelines for reporting results on a timely basis.

In the latest bid for clinical trial transparency, a French government working group has recommended a sweeping plan to publicize study data and issue guidelines for ensuring that results are made available on a widespread and timely basis.
Notably, the working group urged that clinical trial results should be published in a registry within a year after a study is completed. The French National Agency for the Safety of Medicines and Health Products was also encouraged to issue reminders to companies, universities, and others about posting results. And trial sponsors should be urged to incorporate posting obligations in clinical research training.
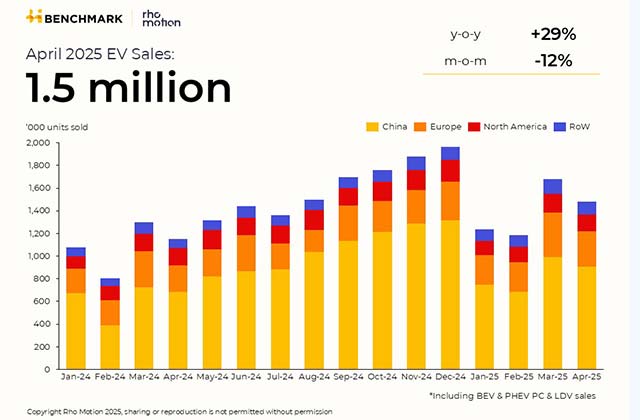
In making its case, the working group noted the French Open Science Monitor, which tracks issues concerning open access to research, found that only 57% of the results of French clinical trials were made public in clinical trial registries, such as Clinicaltrials.gov in the U.S. or the European Union’s registry, between 2012 and 2022.