STAT+: Alnylam enters the ATTR-CM drug race
Today in "The Readout" newsletter: Alnylam's Amvuttra enters ATTR-CM drug race, and Expedition Therapeutics seeks to bring Chinese drugs to the U.S.

Morning! Today, we have plenty of interesting content from yesterday’s STAT Breakthrough Summit East in New York. Also, Alnylam wins an important approval for ATTR-CM.
Alnylam’s Amvuttra enters ATTR-CM drug race
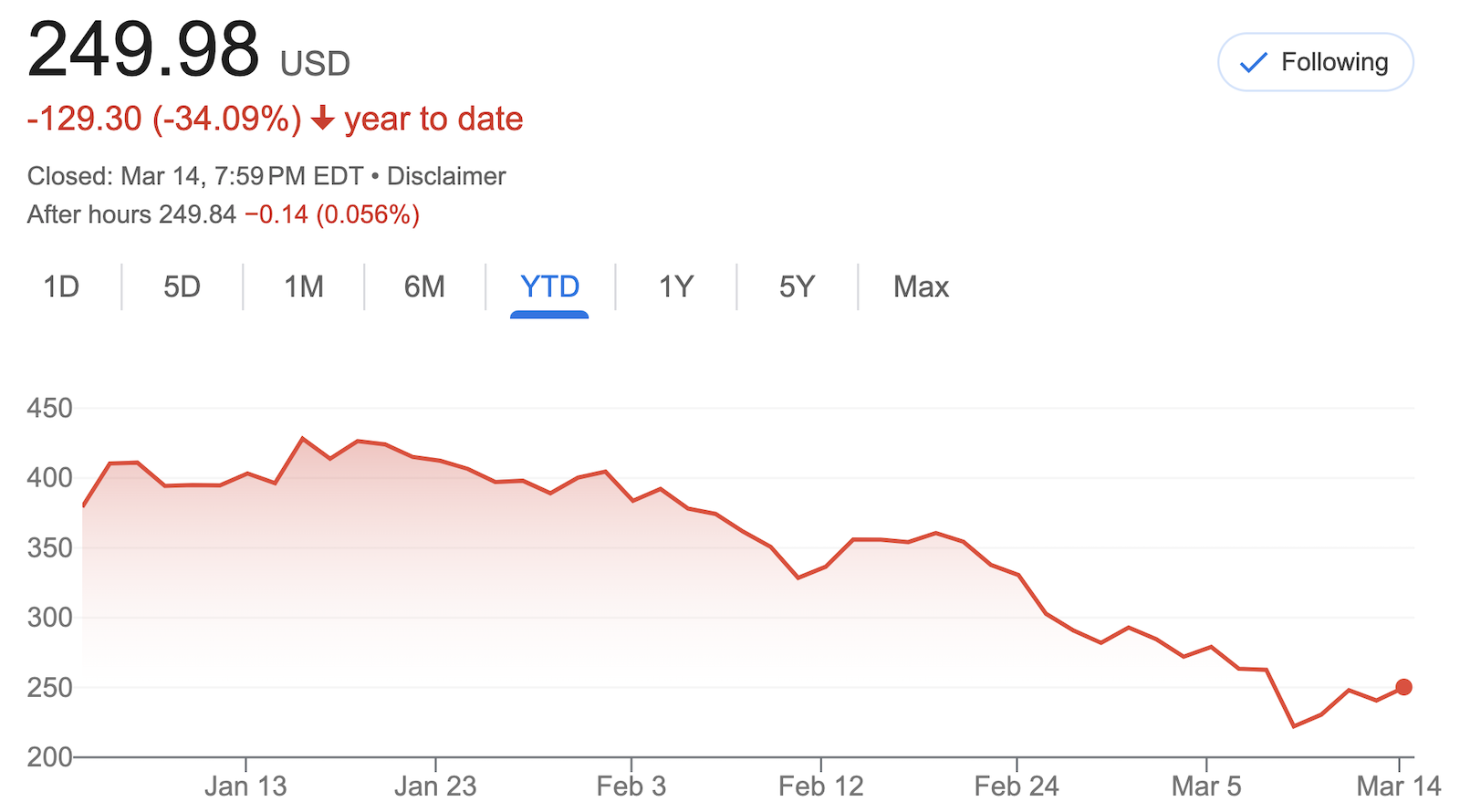
The FDA has approved Alynylam’s RNAi therapy Amvuttra for treating transthyretin amyloid cardiomyopathy (ATTR-CM), positioning it to compete with Pfizer’s Vyndamax and BridgeBio’s Attruby. Priced at $476,000 annually, Amvuttra is significantly more expensive than BridgeBio’s pill and may face access hurdles, particularly with Medicare Advantage.
Unlike its rivals, Amvuttra silences the gene producing unstable proteins rather than stabilizing them. Despite trial data showing efficacy, cross-trial comparisos remain inconclusive. Alnylam sees the launch as a turning point toward profitability, aiming to turn Amvuttra into its flagship product.