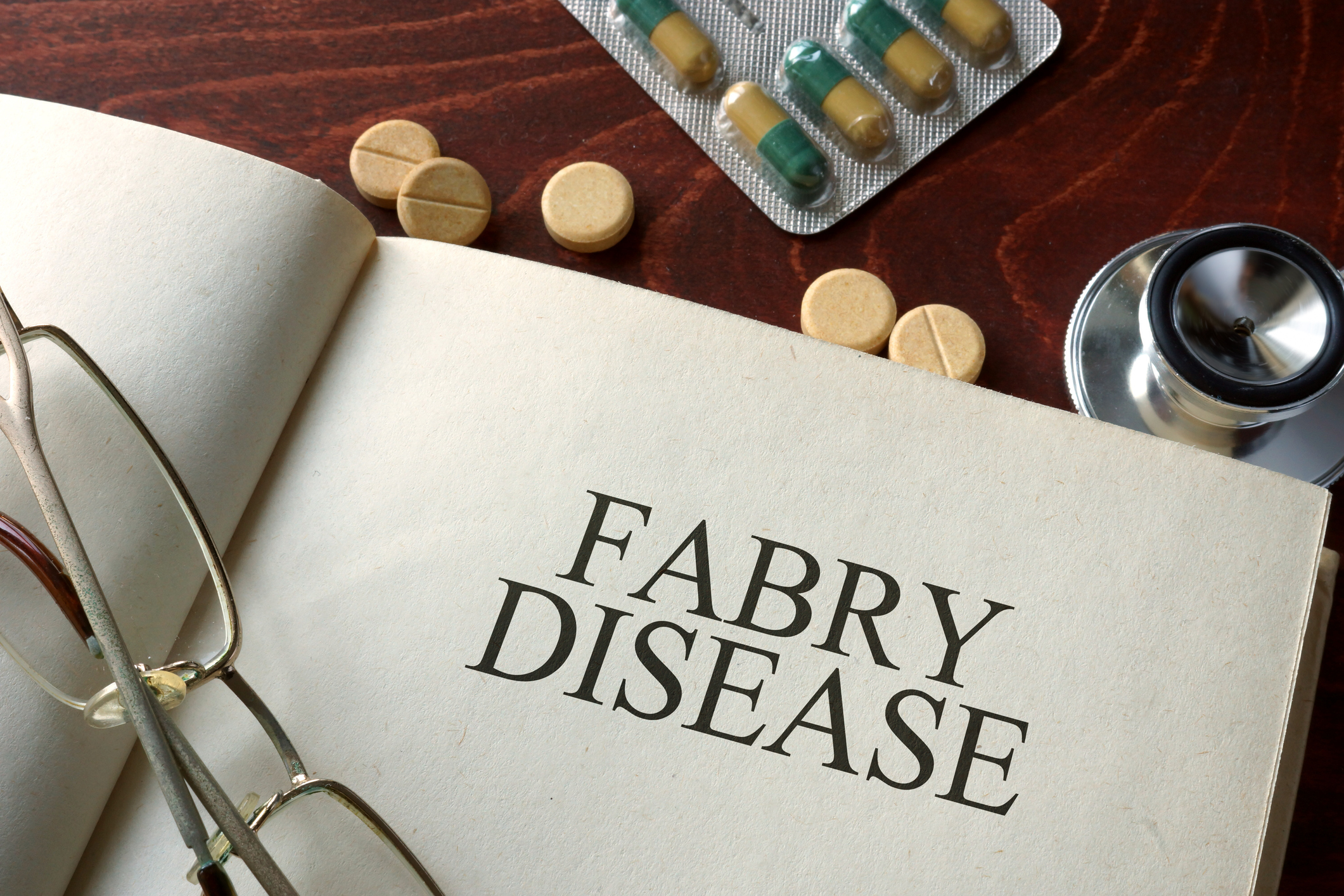
Sangamo preps FDA application for Fabry gene therapy on heels of phase 1/2 registrational data
Sangamo Therapeutics’ investigational gene therapy appears to improve kidney function at 52 weeks for patients with a rare genetic disorder.
Jun 23, 2025 0
Jun 22, 2025 0
Jun 22, 2025 0
Jun 22, 2025 0
Jun 24, 2025 0
Jun 24, 2025 0
Jun 24, 2025 0
Jun 24, 2025 0
Jun 20, 2025 0
Jun 20, 2025 0
Jun 20, 2025 0
Jun 24, 2025 0
Jun 24, 2025 0
Jun 24, 2025 0
Jun 23, 2025 0
Jun 24, 2025 0
Jun 24, 2025 0
Jun 23, 2025 0
Jun 23, 2025 0
Jun 24, 2025 0
Jun 20, 2025 0
Jun 18, 2025 0
Or register with email

May 12, 2025 0
May 15, 2025 0
May 12, 2025 0
May 12, 2025 0
May 22, 2025 0
This site uses cookies. By continuing to browse the site you are agreeing to our use of cookies.
