Enhanced Electrochemical Deuterated Ammonia (ND3) Production via Microenvironment‐Regulated Nitrate Reduction
Advanced Energy Materials, EarlyView.

This study presents an electrochemical approach for efficient deuterated ammonia (ND3) production under ambient conditions. By optimizing the microenvironment and utilizing a highly dispersed iron catalyst, the process achieves high ND3 selectivity and scalability. The results highlight significant economic and environmental advantages over conventional methods, offering a sustainable alternative for industrial-scale ND3 synthesis.
Abstract
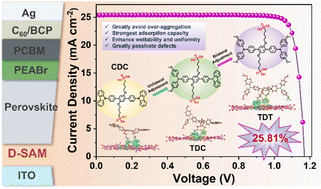
Deuterated ammonia (ND3) has been widely used in pharmaceutical synthesis, chemical analysis, and semiconductor manufacturing, yet its conventional production suffers from high energy consumption, carbon emissions, and prohibitive costs. Here, an electrochemical approach is presented for ND3 production under ambient conditions, achieving industrial-scale performance through microenvironment regulations. Using a highly dispersed iron catalyst on nitrogen doped carbon support for nitrate reduction in D2O, the kinetic limitations of sluggish D2O dissociation are partially mitigated, reaching 80% ND3 selectivity at 200 mA cm⁻2. By identifying key reaction intermediates through in situ spectroscopy and combining their interfacial modulation (via tetraethylammonium, TEA⁺) with deuterium supply optimization (via KD2PO4), ND3 selectivity is further enhanced to 91%. Scaling the process to a 100 cm2 membrane electrode assembly (MEA) type electrolyzer demonstrates viability, yielding 23.1 mmol h⁻1 ND3 at 85% selectivity (2.7 V, 5 A). Techno-economic analysis confirms a 9 fold cost reduction to $6700–8700 per kg ND3 (8–11% of market price), alongside 20–71% lower emissions compared to conventional methods with life cycle analysis (LCA). This work establishes a sustainable, scalable route to ND3 production and addresses energy and environmental challenges in deuterated chemical synthesis.