Pfizer to discontinue its GLP-1 pill for obesity due to liver toxicity
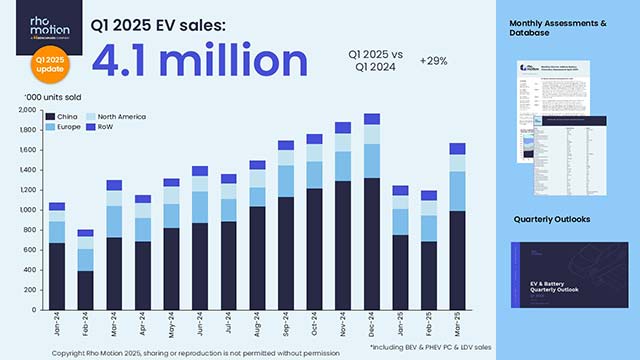
Capping more than two years of stock-gyrating drama, Pfizer said Monday that it would stop development of danuglipron, its experimental oral GLP-1 medicine to treat obesity.

Capping more than two years of stock-gyrating drama, Pfizer said Monday that it would stop development of danuglipron, its experimental oral GLP-1 medicine to treat obesity, focusing its efforts on another medicine with a different mechanism of action.
Pfizer said in a press release that an asymptomatic volunteer in the company’s studies experienced “potential drug-induced liver injury,” which resolved after stopping the medication. After reviewing all clinical data for the medicine and consulting with regulators, Pfizer decided to halt research on it.