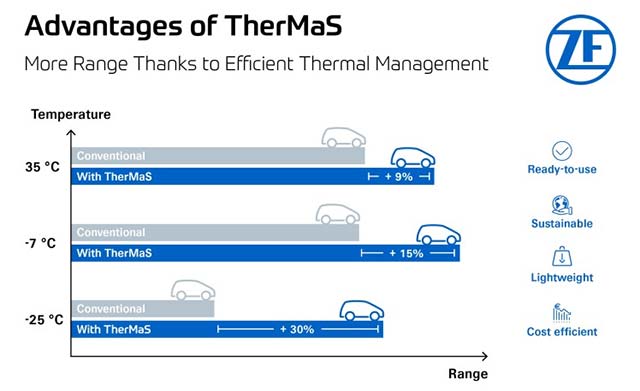
Mg2+‐Loaded Black Phosphorus Nanosheet Protects against Cerebral Ischemic Injury through Anti‐Oxidative and Anti‐Inflammatory Effects
Advanced Healthcare Materials, Volume 14, Issue 13, May 16, 2025.

Dopamine-modified BPNSs loaded with Mg2+ (PBP@Mg), upon injection into the lateral ventricle of MCAO rats, exert anti-apoptotic effects via the mitochondrial pathway by reducing ROS. PBP@Mg also significantly inhibits inflammatory responses by promoting the phenotypic transformation of microglia from M1 phenotype to M2 phenotype in the ischemic penumbra. In summary, PBP@Mg alleviates ischemic brain injury through the aforementioned mechanisms.
Abstract
Ischemic stroke is a severe neurological disease, with high morbidity and mortality worldwide. To date, the treatment of ischemic stroke is limited, and its consequent ischemia-reperfusion injury is an important reason for this result. Excessive reactive oxygen species (ROS) and inflammatory storm followed by ischemia-reperfusion alter the microenvironment of cerebral ischemic penumbra, leading to the devastating damage to the brain. Herein, we design a black phosphorus nanosheets (BPNSs) loaded with magnesium ions (Mg2+) and polydopamine (PBP@Mg) to tackle the above problems. BPNSs of PBP@Mg effectively scavenge excessive ROS in neurocytes. Mg2+ plays an anti-inflammatory role in ischemic penumbra. Furthermore, polydopamine improves the stability of BPNSs. PBP@Mg is subsequently injected into the lateral ventricle of a rat model of ischemic stroke, resulting in an improvement of the ischemic microenvironment and a reduction in ischemic volume. BPNSs of PBP@Mg counteract against the excessive generation of ROS and the neuronal apoptosis in ischemic penumbra. Meanwhile, PBP@Mg dramatically suppresses inflammation by promoting the transformation of microglia from M1 to M2 in ischemic penumbra. PBP@Mg group exhibit a significantly better performance in neurofunctional behavior compared to ischemic group. Taken together, this study provides a novel therapeutic approach for cerebral ischemia-reperfusion injury via anti-oxidative and anti-inflammatory effects.