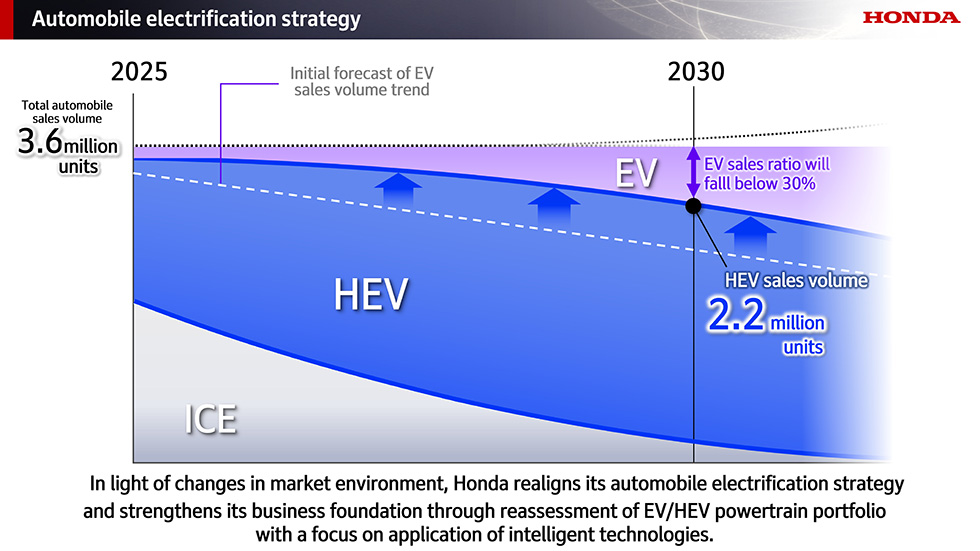
In Situ Monitoring of Dynamic Adsorption‐Induced Interfacial Buffering Toward Highly Stable Zinc Metal Batteries
Advanced Energy Materials, Volume 15, Issue 19, May 20, 2025.

This work presents a real-time monitoring of dynamic interfacial modulation for highly stable zinc metal batteries, employing a theoretically screened cyclen-based additive. Through in situ eQCM characterization and constant-potential MD simulation, the pivotal role of interfacial dynamic adsorption effect is elucidated. The dynamic regulation significantly enhances the stability of interfacial environment and the reversibility of zinc anodes.
Abstract
Electrolyte regulation and electrode/electrolyte interface optimization are recognized as crucial strategies for mitigating parasitic reactions and enhancing zinc plating/stripping in zinc metal batteries. Despite their established importance, the underlying mechanisms of interface behavior and optimization remain elusive, especially in the absence of robust experimental characterization of adsorption-dominated approaches. Herein, in situ monitoring of interfacial adsorption effect is presented, employing a theoretically screened cyclen-based additive. The dynamic adsorption behavior in response to alternating electric fields is identified as pivotal in regulating the metal-electrolyte interfaces, as evidenced by a combination of in situ electrochemical quartz crystal microbalance (eQCM) measurements and constant-potential molecular dynamics simulation. Such dynamic adsorption provides a robust pH buffering effect at the zinc-metal anode interface, facilitating orderly and uniform zinc plating/stripping. Consequently, the electrochemical performance of zinc-based half cells and full cells is markedly enhanced. The findings offer comprehensive insights into the strategic development of functional electrolyte additives for aqueous zinc metal batteries.